Ultrasound guidance allows real-time visualization of the needle in peripheral nerve procedures, improving accuracy and safety. Sonographic visualization of the peripheral nerve and surrounding anatomy can provide valuable information for diagnostic purposes and procedure enhancement. Common procedures discussed are the suprascapular nerve at the suprascapular notch, deep branch of the radial nerve at the supinator, median nerve at the pronator teres and carpal tunnel, lateral cutaneous nerve of the thigh, superficial fibular nerve at the leg, tibial nerve at the ankle, and interdigital neuroma. For each procedure, the indications, relevant anatomy, preprocedural scanning technique, and injection procedure itself are detailed.
Key points
- •
Detailed preprocedural scanning should always be performed before an ultrasound-guided peripheral nerve procedure to determine the ideal approach.
- •
Ultrasound image optimization is necessary for reliably identifying peripheral nerves and appropriately performing procedures.
- •
Having all necessary equipment and planning done in advance will facilitate an effective and safe ultrasound-guided peripheral nerve procedure.
Introduction
Ultrasound guidance allows real-time visualization of the needle in peripheral nerve procedures with improved accuracy and safety. The visualization of the vulnerable target of the peripheral nerve as well as the surrounding anatomy can provide valuable information for both diagnostic purposes and procedure enhancement. Detailed knowledge of the anatomy and appropriate prescanning and equipment preparation can facilitate effective use of ultrasound for peripheral nerve procedures.
Success with peripheral nerve procedures requires knowledge of nerve structure and anatomy, technical skills, and unique challenges associated with peripheral nerves. A thorough knowledge of anatomy, including the peripheral nerve course, function, and surrounding tissue is needed.
Appropriate use of depth, frequency, focal zones, and gray-scale mapping will provide the clearest view of the target region around the peripheral nerve. The depth should be set so the target area takes up the largest portion of the screen. The image should be centered to allow adequate visualization of the approach of the needle to the target. Generally, the highest frequency available that still provides adequate penetration to the tissue desired will create the clearest image of the peripheral nerve. The focal zone should be placed at the desired target for the clearest image ( Fig. 1 ). The gray-scale mapping is chosen to provide the greatest contrast between the nerve and surrounding tissue. Most peripheral nerve injections are performed with a short-axis view of the nerve and an in-plane view of the needle. This allows the best view of the outline of the nerve and visualization of the approach of the needle. It some situations, it is advantageous to rotate between short-axis and long-axis views of the nerve to establish both vantage points.

The patient should be positioned between the clinician and the ultrasound screen to allow easy visualization of both the needle at the target site and the ultrasound image. The necessary equipment for the injection should be reviewed before the procedure and placed easily within reach. Detailed preprocedural scanning should be performed before the procedure to plan the approach and investigate the surrounding anatomy, including potential anatomic variants.
Peripheral nerves provide unique challenges as an injection target, including borders that can be somewhat indistinct relative to surrounding tissue. Nerves also are relatively mobile and have the potential to move from the initial target site with tissue movement as well as infiltrating injectate. Nerves are also vulnerable targets with considerable potential for injury. Some investigators argue that intraneural injections are relatively safe if the needle does not penetrate the fascicles. Despite this, caution is recommended for all injections because of limits of resolution and variability of the pattern of fascicular architecture in some nerves. Creating a halo around the nerve with injectate can increase the conspicuity of nerve borders ( Fig. 2 ). Use of blunt needles can also help facilitate safe injections very close to the peripheral nerve borders.
Introduction
Ultrasound guidance allows real-time visualization of the needle in peripheral nerve procedures with improved accuracy and safety. The visualization of the vulnerable target of the peripheral nerve as well as the surrounding anatomy can provide valuable information for both diagnostic purposes and procedure enhancement. Detailed knowledge of the anatomy and appropriate prescanning and equipment preparation can facilitate effective use of ultrasound for peripheral nerve procedures.
Success with peripheral nerve procedures requires knowledge of nerve structure and anatomy, technical skills, and unique challenges associated with peripheral nerves. A thorough knowledge of anatomy, including the peripheral nerve course, function, and surrounding tissue is needed.
Appropriate use of depth, frequency, focal zones, and gray-scale mapping will provide the clearest view of the target region around the peripheral nerve. The depth should be set so the target area takes up the largest portion of the screen. The image should be centered to allow adequate visualization of the approach of the needle to the target. Generally, the highest frequency available that still provides adequate penetration to the tissue desired will create the clearest image of the peripheral nerve. The focal zone should be placed at the desired target for the clearest image ( Fig. 1 ). The gray-scale mapping is chosen to provide the greatest contrast between the nerve and surrounding tissue. Most peripheral nerve injections are performed with a short-axis view of the nerve and an in-plane view of the needle. This allows the best view of the outline of the nerve and visualization of the approach of the needle. It some situations, it is advantageous to rotate between short-axis and long-axis views of the nerve to establish both vantage points.
The patient should be positioned between the clinician and the ultrasound screen to allow easy visualization of both the needle at the target site and the ultrasound image. The necessary equipment for the injection should be reviewed before the procedure and placed easily within reach. Detailed preprocedural scanning should be performed before the procedure to plan the approach and investigate the surrounding anatomy, including potential anatomic variants.
Peripheral nerves provide unique challenges as an injection target, including borders that can be somewhat indistinct relative to surrounding tissue. Nerves also are relatively mobile and have the potential to move from the initial target site with tissue movement as well as infiltrating injectate. Nerves are also vulnerable targets with considerable potential for injury. Some investigators argue that intraneural injections are relatively safe if the needle does not penetrate the fascicles. Despite this, caution is recommended for all injections because of limits of resolution and variability of the pattern of fascicular architecture in some nerves. Creating a halo around the nerve with injectate can increase the conspicuity of nerve borders ( Fig. 2 ). Use of blunt needles can also help facilitate safe injections very close to the peripheral nerve borders.
General procedural considerations
A detailed discussion of all peripheral nerve procedures is beyond the scope of this article. The more commonly performed procedures are discussed. There are often multiple approaches to performing peripheral nerve injections. The following represent the author’s preferred techniques. In all of the following injections, a short-axis view of the nerve and in-plane view of the needle are preferred.
A high-frequency (>10 MHz) linear-array transducer is best for visualization of superficial structures and should be used for most peripheral nerve injections ( Fig. 3 ). Peripheral nerve injections always should be performed with sterile technique, including use of appropriate sterilizing agents for the skin. Alcohol-based chlorhexidine for this purpose is preferred over povidone-iodine by many institutions. Use of sterile probe covers can alleviate the need to sterilize the transducer surface ( Fig. 4 ). This is generally preferred because it allows free movement of the transducer in the field for optimization of tissue and needle conspicuity. Transducer covers were not used for most of the images in this article for picture clarity.
The transducer always should be maintained in as close to an orthogonal position relative the needle as possible. This will provide the highest conspicuity of the needle. With an in-plane approach, ensure that the needle remains in parallel underneath the transducer. Approach at an oblique angle will result in visibility of only a portion of the needle. The needle should not be advanced if the tip is not visible. The needle direction of many peripheral nerve injections is relatively superficial. Use of an oblique gel stand-off can assist with establishing proper needle trajectory before entry through the skin. This is accomplished by using a large amount of sterile gel on the needle entry side of the transducer.
The injectate used for each procedure is based on the desired intervention. Local anesthetics alone in a volume of 1 to 4 mL are typically used for most nerve blocks. Injectable corticosteroids are often used in conjunction with the anesthetic if the goal is longer relief, particularly in the context of entrapment neuropathies. The flow of the injectate should always be initiated slowly and watched carefully to ensure proper location because of the potential vulnerability of the target. The nerve is often more conspicuous once the initial injectate is placed near the target. Larger volumes of injectate are used for hydrodissection. This can consist of 5 to 15 mL of a combination of normal saline and local anesthetic. Some also use dextrose solution. The literature regarding hydrodissection techniques and whether they provide significant benefit over standard perineural injections is sparse at this time, and further studies are needed to provide better insight into the appropriate indications and methods for this technique.
Suprascapular Nerve at the Suprascapular Notch
Indications
Suprascapular nerve injections are most often used to treat intractable shoulder pain. Acutely this often is for postoperative shoulder pain. In the context of chronic shoulder pain, the injections can be used in a context of a diagnostic trial or with longer-acting agents, including anesthetic agents with steroids, toxic agents such as phenol, and radiofrequency ablations. Most injections of the suprascapular nerve are performed at the suprascapular notch. Some investigators advocate a supraclavicular approach. Aspiration of compressive posterior labral cysts are also performed in the area of the spinoglenoid foramen ( Fig. 5 ).
Anatomy
The suprascapular nerve arises from the C5 and C6 roots and departs the brachial plexus near the location of the convergence of those roots as they form the superior trunk. The nerve travels deep to the trapezius toward the posterior shoulder along the border of the scapula. It becomes visible on ultrasound as it traverses through the suprascapular notch deep to the superior transverse scapular ligament. It is accompanied by the suprascapular artery and vein. It then gives branches to the supraspinatus in the supraspinatus fossa and continues around the lateral border of the spine of the scapula into the infraspinatus fossa. From there it gives branches to the infraspinatus. The suprascapular nerve provides motor innervation to the supraspinatus and infraspinatus as well as sensory innervation to the acromioclavicular and glenohumeral joints.
Scanning
The suprascapular nerve can be visualized with the suprascapular artery and vein at the suprascapular notch and also at the spinoglenoid foramen ( Fig. 6 ). The transducer is placed in the plane of the spine of the scapula. Internal and external rotation of the shoulder as well as use of Doppler imaging may help distinguish the suprascapular artery and vein. The hypoechoic suprascapular vein will enlarge and collapse with internal and external rotation. Effort should be made to identify the superior transverse ligament at the suprascapular notch; however, some investigators have suggested that this may be difficult to distinguish sonographically from the fascia of the supraspinatus muscle.
Procedure
- a.
Needle: 22 gauge, 64 to 89 mm ( Figs. 7 and 8 ).
Fig. 7
Arrangement for an injection near the suprascapular nerve at the suprascapular notch.
Fig. 8
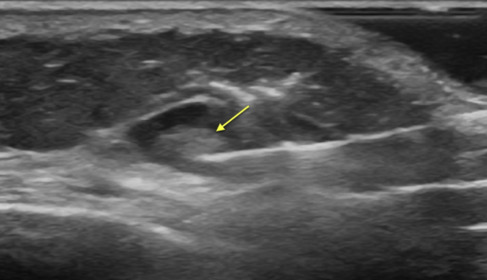
Sonogram demonstrating the needle approach ( arrow ) for an injection near the suprascapular nerve at the suprascapular notch.
- b.
Patient position:
- i.
Seated with the hand on the contralateral shoulder; or
- ii.
Prone with the arm hanging off the edge of the table.
- i.
- c.
Transducer position: Parallel to the spine of the scapula over the suprascapular notch.
- d.
Needle approach: Medial-to-lateral or lateral-to-medial are both effective.
- e.
Target: Near the suprascapular nerve at the suprascapular notch. The needle should be directed deep to the superior transverse scapular ligament.
- f.
Avoid: Intravascular injection of the suprascapular artery and vein.
- g.
Tip: There is often temporary resistance when passing through the superior transverse scapular ligament.
Deep Branch of the Radial Nerve at the Supinator
Indications
Entrapment of the deep branch of the radial nerve at the supinator is also known as radial tunnel syndrome. It is a controversial source of forearm pain and is considered a possible pain generator in recalcitrant lateral epicondylalgia. Measurable neurologic deficit from this location is rarely reported in the absence of trauma or mass. The literature is currently sparse respecting the efficacy of this injection.
Anatomy
The radial nerve bifurcates into the deep branch of the radial nerve and the superficial radial sensory nerve near the level of the radiocapitellar joint. The deep branch of the radial nerve enters the radial tunnel through the Arcade of Frohse (supinator arch), which is the fibrous entrance to the space between the superficial and deep layers of the supinator ( Fig. 9 ). The nerve becomes the posterior interosseus nerve once it exits the supinator and lies on the posterior interosseus membrane. It innervates the extensor digitorum, extensor digiti minimi, extensor carpi ulnaris, abductor pollicis longus, extensor pollicis longus, extensor pollicis brevis, and extensor indicis. Significant injury to this nerve can result in weakness of digit extension and radial deviation of the wrist with extension. The actual entrapment site in this area is controversial. Many propose that the nerve is typically entrapped as it enters the Arcade of Frohse. Others support entrapment in or at the exit point of the radial tunnel.
Scanning
The deep branch of the radial nerve can be identified as it bifurcates from the superficial sensory branch near the elbow joint. It is followed in short-axis toward its entrance to the supinator. The recurrent radial artery and its accompanying veins (Leash of Henry) can be identified before the entrance of the nerve through the Arcade of Frohse. The nerve should then be followed through the exit from the supinator. It should be assessed in both short-axis and long-axis for both focal and diffuse enlargement along its course. Comparison with the presumably unaffected contralateral side should always be done when enlargement is suspected and the precise location of the procedure is being determined ( Fig. 10 ).
Procedure
- a.
Needle: 25 gauge to 27 gauge, 51 to 89 mm ( Figs. 11 and 12 ).
Fig. 11
Arrangement for an injection near the deep branch of the radial nerve at the supinator.
Fig. 12
Sonogram demonstrating the needle approach ( long arrow ) for an injection near the deep branch of the radial nerve ( short arrows ) in short-axis at the supinator.
- b.
Patient position: Supine or seated with the forearm resting on the table, the elbow slightly flexed, and the thumb pointed upward.
- c.
Transducer position: Short-axis for injection. Both short-axis and long-axis often used for hydrodissection.
- d.
Needle approach: Medial-to-lateral for single injection. Use of medial-to-lateral and also distal-to-proximal for hydrodissection.
- e.
Target: The deep branch of the radial nerve near an identified focal flattening and proximal swelling. Often between the superficial and deep heads of the supinator.
- f.
Avoid: Intraneural injection and injury to the recurrent radial artery.
Median Nerve at the Pronator Teres
Indications
Entrapment of the median nerve at the level of the pronator teres is also known as “pronator tunnel syndrome.” It is a rare source of median neuropathy and should be considered when there is neurologic deficit involving the median-innervated forearm muscles or pain in the median nerve distribution that is not attributed to median neuropathy at the carpal tunnel. It is reported to present in the absence of neurologic deficit as a vague ache in the forearm that is worsened by repetitive motion.
Because the pronator teres is not a typical entrapment site, such as the much more common carpal tunnel, detailed use of history, physical examination, and when appropriate, electrodiagnosis and diagnostic imaging should be used to confirm focal neuropathy in this location.
Anatomy
The median nerve is supplied by the cervical roots of C6 to T1. The median nerve traverses the elbow medial to the nearby brachial artery. It passes beneath the lacertus fibrosus and the radial head of the pronator teres, and then between the radial and ulnar heads of the pronator teres. In most individuals, the ulnar artery emerges as a bifurcation from the brachial artery and traverses posterior to the median nerve at the level of the pronator tunnel. The innervations for the flexor carpi radialis, palmaris longus, and flexor digitorum superficialis emerge near the level of the pronator teres. The anterior interosseus nerve typically emerges from the main trunk of the median nerve just after exiting the pronator teres. The main trunk of the median nerve continues distally to innervate the abductor pollicis brevis, opponens pollicis, superficial head of the flexor pollicis brevis, and the first and second lumbricals. It additionally provides cutaneous sensation to the palm and palmar aspect of the thumb, index, and long fingers, as well as the radial side of the ring finger.
Scanning
The nerve should be followed in short-axis view from above the antecubital fossa through the heads of the of the pronator teres ( Fig. 13 ). The nerve also should be assessed in long-axis ( Fig. 14 ). A heel-toe maneuver with the transducer can reduce anisotropic artifact in the long-axis image. The nearby brachial artery and its ulnar artery branch should be identified for avoidance during the injection.
Procedure
- a.
Needle: 25 to 27 gauge, 38 to 64 mm ( Figs. 15 and 16 ).
Fig. 15
Arrangement for an injection near the median nerve at the level of the pronator tunnel.
Fig. 16
Sonogram demonstrating the needle approach ( long arrow ) for an injection near the median nerve in short-axis at the level of the pronator tunnel.
- b.
Patient position: Seated or supine with the forearm resting on the table in supinated position and the elbow relatively extended.
- c.
Transducer position: Short-axis for injection. Both short-axis and long-axis often used for hydrodissection.
- d.
Needle approach: Medial-to-lateral for single injection. Use of medial-to-lateral and also distal-to-proximal for hydrodissection.
- e.
Target: The median nerve near an identified focal flattening and proximal swelling. Often between the radial and ulnar heads of the pronator teres.
- f.
Avoid: An intraneural injection of the median nerve. Also identify and avoid the more lateral brachial artery proximally and the bifurcation and path of the ulnar artery at the more distal path of the median nerve. Identification of the anterior interosseus nerve and artery is needed when injecting at the more distal aspect of the pronator tunnel.
Median Nerve at the Carpal Tunnel
Indications
Entrapment of the median nerve at the carpal tunnel is the most common and well-defined neuropathy in the human body and is known as carpal tunnel syndrome. Clinical, electrophysiologic, and imaging techniques facilitate diagnosis of this condition with a high degree of accuracy. Use of localized steroid injections have been shown to be an effective intervention for providing symptomatic relief. There has been growing interest in the use of hydrodissection for this condition.
Anatomy
The carpal tunnel space is bordered by the carpal bones on the radial, ulnar, and dorsal sides. The volar aspect is covered by the transverse carpal ligament. The carpal tunnel also contains the tendon of the flexor pollicis longus, the 4 flexor digitorum superficialis tendons, and the 4 flexor digitorum profundus tendons. The ulnar tunnel lies to the ulnar side of the wrist and contains the ulnar artery and nerve ( Fig. 17 ). The median nerve enters the carpal tunnel space at the carpal tunnel inlet. This is identified on imaging with the bony landmarks of the scaphoid, lunate, and pisiform ( Fig. 18 ). This is frequently the site of abnormal enlargement of the median nerve. In neuropathy at the carpal tunnel, the nerve is typically flattened at the carpal tunnel outlet where the transverse carpal ligament is generally thicker ( Fig. 19 ). The carpal tunnel outlet is identified by the bony landmarks of the trapezium on the radial side and hamate on the ulnar side.








