Tremor and other involuntary movement
Wendy Romney, Michelle E. Wormley and Michelle M. Lusardi
Introduction
Many of the neuromuscular diseases common with aging have signs and symptoms that include extraneous or involuntary movement. Some have little impact on functional ability whereas others can significantly compromise an older adult’s ability to safely or efficiently accomplish functional tasks. In order to select the most appropriate measures of impairment and function, and to develop a plan of care that will enhance safety and function, rehabilitation professionals need to differentiate between the possible causes, characteristics and management of the various involuntary movements and dyskinesias encountered when working with older adults. In this chapter, we define the most common types of dyskinesia, present a classification of movement dysfunction and review the evidence for examination and functional interventions in individuals who exhibit involuntary movement.
Definition of terms
Dyskinesia
The term dyskinesia is used when extraneous or unintended motion is routinely observed during postural and/or functional tasks. Tremor is the most common form of dyskinesia. Other forms of dyskinesia include: dystonia, clonus, choreoathetosis and ballism. Dyskinesia occurs at various levels within the central nervous system (CNS). Dystonia (fixed abnormal postures) and clonus (recurrent hyperactive deep-tendon responses to sudden changes in muscle length) are common in diseases affecting the pyramidal (voluntary) motor systems. Tremors at rest, writhing choreoathetosis and ballism suggest impairment in the extrapyramidal system at the level of the basal ganglia. Tremors that increase in severity with movement often indicate cerebellar dysfunction. Fasciculation, often mistaken for tremor, occurs as a result of an adverse drug reaction or denervation.
Tremor
Tremor, the most prevalent involuntary movement, is characterized by a rhythmic oscillation around a fixed axis, often congruent with the axis of motion of the affected joint or joints (Alty & Kempster, 2011). The frequency (period) and waveform (timing, sequence of muscle activity) of a particular type of tremor is remarkably consistent over time, although the amplitude of the tremor may vary with internal factors (e.g. fatigue, anxiety, stress, emotions) or external factors (e.g. ambient temperature, alcohol or other substance use, environmental conditions or demands) (Bhidayasiri, 2005).
Tremor appears to be the result of alternating contraction of muscles on either side of a joint. The underlying CNS mechanisms of tremor are not clearly understood; there are several interactive factors that may contribute to the motor expression of tremor: the oscillating tendencies of the mechanical systems of joints and muscles; short- and long-loop spinal cord and brainstem reflexes; closed-loop feedback systems of higher motor centers, including the cerebellum.
Identifying when a tremor occurs (activation) is one strategy for classification: tremor may occur only during movement (action tremor), only when at rest (resting tremor), when trying to maintain a relatively fixed posture (postural tremor), or under all of these conditions. Other strategies for identifying tremor involve observing anatomical location, frequency and amplitude. Most tremors increase with higher levels of stress, anxiety or fatigue and decrease or disappear during periods of sleep (Daroff et al., 2012).
Fasciculation
Fasciculation is spontaneous discharge from whole or partial motor units, that may be mistaken for tremor (Daroff et al., 2012). On careful observation, fasciculations present as random twitching rather than the rhythmic oscillating contraction seen in tremor. Fasciculation can occur in motor neuron diseases like amyotrophic lateral sclerosis or primary lateral sclerosis. Fasciculation may also be seen as a result of anticholinergic drugs and stimulants (e.g. excessive caffeine), electrolyte imbalance or sodium deficiency, muscle denervation, nerve root irritation (herniated intervertebral disc or spondylosis). Fasciculation can sometimes be observed in periods of extreme stress or fatigue, or following excessive strenuous exercise.
Myoclonus
Myoclonus (clonus) is sudden, brief involuntary movement caused by muscular contractions (positive myoclonus) or inhibitions (negative myoclonus). Due to its rhythmic involuntary nature, it can resemble tremor (Weiner & Lang, 2005). Myoclonus occurs under three circumstances:
3. less commonly, as a component of a familial, idiopathic or physiologically induced movement disorder (Blumenfeld, 2010).
Myoclonus associated with hyperactive stretch reflexes can be transient (lasting for several beats) or sustained over a period of time (mimicking tremor). It can be ‘triggered’ by rapid elongation of affected muscles, as in deep-tendon reflex testing; rapid passive range of motion (commonly examined at the ankle with a quick stretch of the gastroc-soleus by dorsiflexion); or during position change. The peripheral mechanism of myoclonus is the same as that of the stretch reflex: annulospiral ‘endings’ around intrafusal fibers within the muscle spindle are stimulated by elongation of muscle tissue. Information about change in length is carried to the CNS via 1a afferent neurons in peripheral nerves. These 1a neurons synapse directly with alpha-motor neurons in the anterior horn of the spinal cord or motor cranial nerve nuclei. If stimulated sufficiently, alpha-motor neurons trigger the activation of the motor units of the elongated extrafusal muscle. The resulting contraction elongates the antagonistic muscles on the other side of the joint, triggering the stretch reflex. Deep tendon reflexes are classified as follows: 0 (absent), 1+(hypoactive), 2+(normal), 3+(hyperactive response without clonus) and 4+(hyperactive response with myoclonus) (Paz & West, 2008). Many individuals with myoclonus associated with pyramidal system dysfunction also exhibit a positive Babinski response when the lateral plantar surface of the foot is stimulated (an upward-pointing hallux with fanning of the second to the third toes).
Myoclonus observed during seizures may involve a single limb segment (in a partial seizure of the opposite motor cortex) or rhythmic jerking of multiple limbs (in a generalized tonic–clonic seizure of the entire cortex). The combination of altered consciousness and myoclonus differentiates the involuntary movement of seizures from tremor. An electroencephalogram (EEG) recorded during either partial or generalized seizure demonstrates abnormal electrical activity of the motor cortex, whereas EEG patterns in those with tremor are less likely to be grossly abnormal (Raethjen et al., 2007; Muthuraman et al., 2008; Shibasaki, 2012).
Hiccups and ‘sleep starts’ (nocturnal myoclonus) are examples of physiologically triggered myoclonus. Movement-triggered myoclonus has been reported during recovery from severe cerebral hypoxia or ischemia following myocardial infarction or near drowning. Myoclonus may occur as a component of uremic or hepatic encephalopathy and with degenerative disorders, as in Alzheimer’s disease. Occasionally, myoclonus may be caused by drug toxicity (e.g. penicillin, tricyclic antidepressant, levodopa) (Rowland & Pedley, 2010).
Tics
Tics are brief and intermittent movements (motor tics) or sounds (phonic tics) that can resemble myoclonus and tremor as well as the dance-like involuntary movement of chorea (Daroff et al., 2012). Tics can be classified as ‘simple’, involving brief irregular muscle twitching of an isolated body segment, as in the case of repetitive eye blinking, throat clearing, or shoulder shrugging. Tics can also present as ‘complex’, for example in the case of coordinated, patterned movements involving several muscles, as in arm gesturing, skipping while walking, and whistling or stuttering (Rowland & Pedley, 2010; Daroff et al., 2012). Those experiencing tics will describe a sense of increasing muscle tension that can only be relieved when the stereotypical movement occurs. Tics differ from other types of involuntary movement in that they are somewhat under voluntary control and can be suppressed for a length of time. Idiopathic tics often occur for short periods of time, sometimes in childhood, and may be associated with anxiety or other psychological stress factors. Tics differ from other dyskinetic movement disorders as they may be evident during all stages of sleep, although they may subside with sleep. Tics associated with Tourette’s syndrome may persist over the lifespan and include vocalizations (barking, grunting, echolalia and repetitive swearing) as well as stereotypical facial or extremity movement.
Dystonia
Dystonia is a movement disorder characterized by a sustained positioning or a very slowly changing abnormal synergistic movement (Alarcon et al., 2004). It can affect one or more body segments, often observed as tonic abnormal posturing in individuals with longstanding damage to the pyramidal motor system (e.g. severe equinovarus after significant stroke or other acquired brain injury, or spastic cerebral palsy). Dystonic positions are described as abnormal; they cannot be accurately mimicked or recreated volitionally. Individuals with dystonia associated with pyramidal system dysfunction may also exhibit myoclonus and hypertonicity.
Some dystonias are idiopathic and may be familial (e.g. spastic torticollis). Others occur only during one specific motor activity (e.g. writer’s cramp or laryngeal dystonia during public speaking). Facial hemispasm is an intermittent focal dystonia related to compression or irritation of the seventh cranial nerve. If idiopathic torsion dystonia develops in later life, it most commonly affects axial, facial or upper extremity muscles and may challenge feeding, communication and other activities of daily living (ADLs). Most idiopathic dystonias are nonprogressive.
Secondary dystonias may be associated with damage to the putamen nucleus of the basal ganglia resulting from a tumor, ischemia or infarct, or head injury. Dystonia may be one of the signs of progressive degenerative diseases such as supranuclear palsy, Huntington’s disease, Wilson’s disease or Parkinson’s disease. Dystonic postures may emerge in the end-stages of Alzheimer’s disease.
Medications used to manage dystonia and spasticity must be closely monitored owing to adverse side-effects. Medications include: benzatropine mesylate (Cogentin), diazepam (Valium), dantrolene (Dantrium), haloperidol (Haldol), baclofen (Lioresal, Clofen), tizanidine hydrochloride (Zanaflex), carbamazepine (Tegretol) and Gabapentin (Neurontin) (Ciccone, 2007; Gladson, 2010). Severe focal dystonia may be temporarily treated with injection of botulinum toxin.
Chorea
Chorea is a less common dyskinesia consisting of the random and rapid involuntary contractions of muscle groups, mostly of the extremities or face (Rowland & Pedley, 2010). Proximal and/or distal muscle groups of the extremities may be affected. Typically, muscles of the axial skeleton are not involved; therefore, postural control is not significantly compromised.
Chorea occurs when there is damage to the corpus striatum (basal ganglia), especially the caudate nucleus and putamen; however, the exact localization and pathophysiology of chorea is uncertain (Patestas & Gartner, 2006). Some choreas are hereditary (e.g. Huntington’s disease), whereas others are a consequence of another physiological disease or trauma (Blumenfled, 2010). Choreic movement also occurs with tardive dyskinesia, a complication of the long-term use of certain neuroleptic drugs (e.g. in the management of schizophrenia) or dopamine toxicity (e.g. in the management of Parkinson’s disease) (Caligiuri et al., 2000).
The quality of choreiform movement is often described as graceful or dance-like. Individuals learn to blend their involuntary movement with a purposeful movement in an attempt to mask or minimize the unwanted movement (e.g. a choreic movement of the arm over the head might be turned into smoothing of the hair). People with chorea will often have difficulty sustaining contractions (e.g. ‘milk-maid’s handshake’ – the patient contracts and relaxes when asked to maintain a constant, firm grip during a handshake). As with tremor, choreiform movements become more obvious in periods of stress and may disappear during sleep. Pseudochorea has been reported in individuals with impairment of proprioception resulting from multiple sclerosis and other diseases of the dorsal columns.
Athetosis
Athetosis is a continuous, slow, involuntary, writhing movement. Athetosis is mostly observed in muscles of the extremities (distal to proximal) but it can also involve muscles of the face, neck and postural muscles of the trunk. It may be associated with dystonic postures, chorea or spasticity. Athetoid movements that are brief may be associated with chorea (choreoathetosis). Writhing movements that are sustained, may be associated with dystonia (athetotic dystonia). Individuals with athetosis have difficulty sustaining positions at rest and during volitional movement. Athetosis affects the efficacy of postural control when sitting and standing, as well as during both transitional and skilled movements necessary for mobility and activities of daily living. Athetosis is slower and less jerky than chorea and unable to be sustained, as with dystonia.
Athetosis occurs when there has been damage to the corpus striatum (caudate and putamen) in the basal ganglia, most often in children with perinatal ischemia and hypoxia or severe bilirubin toxicity. In the past, athetosis was referred to as the basal ganglia form of cerebral palsy. Currently, the term dyskinetic cerebral palsy is preferred and the use of athetosis is recommended only to indicate a particular type of movement independent of etiology. Although the severity of athetosis does not change with maturity, function may become more challenging in aging individuals with athetosis because of typical age-related changes and increased incidence of musculoskeletal and neuromuscular pathologies that are common later in life. Pseudoathetosis may occur in adulthood due to severe distal sensory loss (Spitz et al., 2006).
Ballismus or ballism
Ballismus is a rarely occurring movement disorder that presents as wild and forceful flinging movements of one or more of the extremities that are rapid and nonpatterned (Arminoff, 2008; Klein, 2005). Trunk and facial muscles are usually spared and bulbar functions (e.g. speaking, swallowing, breathing) are not impaired. Ballismus is usually unilateral (hemiballismus) and movements are much more stereotypical and disruptive than those seen in chorea. It may be suggested that ballism and chorea represent a continuum rather than distinct entities (Daroff et al., 2012). Ballismus differs from other dyskinesias in that these involuntary motions do not tend to decrease in frequency or amplitude during periods of sleep.
Ballismus is thought to occur when there has been damage or disruption to the subthalamic nuclei in the diencephalon. Alteration of neural output from the subthalamus apparently ‘releases’ the activity of the globus pallidus nuclei, which unleashes stereotypical synergistic movement of the limb girdle and extremity. It occurs most often as the result of a ‘lacunar’ stroke of the lenticulostriate branches of the middle cerebral artery, which damages the subthalamus deep in one cerebral hemisphere. Haloperidol (Haldol) is often used to control unwanted and disruptive motion during the acute and early rehabilitation phases of care, and to promote more effective sleeping. Fortunately, hemiballistic movement tends to diminish in both amplitude and frequency in the weeks following a stroke; however, more subtle choreoathetotic movements may persist (Blumenfled, 2010).
Asterixis
Asterixis, also referred to as negative myoclonus, occurs as a brief and recurrent loss of sustained muscle contractions in postural muscles of the extremities and trunk (Rubboli & Tassinari, 2006; Rowland & Pedley, 2010). Asterixis is observed during neurological examination when the person being assessed exhibits ‘flapping’ of the hands when asked to hold their arms horizontally with wrists extended against gravity. Asterixis may occur in individuals with toxic metabolic encephalopathy as a result of hepatic, renal or pulmonary disorders. It has also been reported as a consequence of drug toxicity, during anticonvulsant therapies and when there is a lesion interrupting interconnections between the brainstem and thalamus.
Akathisia (restless leg syndrome)
Akathisia, often called restless leg syndrome, is a distressing subjective sense of tension and discomfort of the limbs that is often associated with agitation and a need to move around, but that is not always relieved by movement (Weiner & Lang, 2005). Restless leg syndrome occurs in 10–35% of people over the age of 65 (Milligan & Chesson, 2002). Those with the clinical diagnosis of akathisia report difficulty sitting or lying still and a powerful urge to move. They may pace or rock in place and often complain of difficulty sleeping. Akathisia can be idiopathic or can be an extrapyramidal side-effect of antipsychotic medication. It may be the presenting symptom in someone who is developing tardive dyskinesia (see Drug-induced Movement Disorders below). The FDA has approved three drugs for the management of restless leg syndrome, including pramipexol (Mirapex), ropinirole (Requip) and gabapentin enacrabil (Neurontin Horizan) (Milligan & Chesson, 2002).
Classification and differential diagnosis of tremors
Neurologists and therapists use a variety of subjective and observed characteristics when examining the movement dysfunction of individuals who experience tremor (Bhidayasiri, 2005; Alty & Kempster, 2011; Crawford & Zimmerman, 2011). These factors include: when the tremors occur, the frequency and amplitude, the body segments affected by the tremor, whether there is a family history, their responsiveness to medications and their association with additional CNS signs and symptoms (Table 31.1).
Table 31.1
Comparison of classification strategies for tremor
| Type of Tremor | Frequency (cps)a | Behavior | Characteristics | Response to Medication |
| Normal physiological | 7–12 | At rest | Not observable without instrumentation | Increases with sympathetic activity |
| Enhanced physiological | 7–11 | Action | Low amplitude, visible bilaterally with outstretched arms, increases with anxiety, stress | Increases with certain medications and metabolic conditions; uncommonly treated |
| Essential | 8–10 | Postural, action | Symmetric, involves hands and wrists (handwriting large and tremulous), lower extremities, head or voice; may improve with alcohol, prevalence increases with advanced age | Decreases with beta blockers, primidone, thalamic DBS |
| Parkinsonian tremor | 4–5 | Rest | Asymmetric, involves distal extremities (handwriting small and illegible), decreased with voluntary movement; other signs include bradykinesia, postural instability and rigidity | Decreases with anticholinergics, amantadine, MAO inhibitors, catechol- O-methyltransferase inhibitors and levodopa, dopamine agonists, thalamic DBS |
| Intention/ cerebellar | 3–5 | Action, postural | Ispilateral involvement to lesion, amplitude increases with intention. Other signs: abnormal finger to nose, ataxia, abnormal heel to shin test, hypotonia | Decreases with dansetron, isoniazid, physostigmine, carbamazepine, clonazepam, thalamic DBS |
| Psychogenic | 4–10 | Rest, postural or action | Abrupt onset, spontaneous remission, extinction with distraction, involves both arms, head and lower extremities, absence of other neurological signs, associated with stressful life event | May be referred to psychiatry services for identification and management of illness |
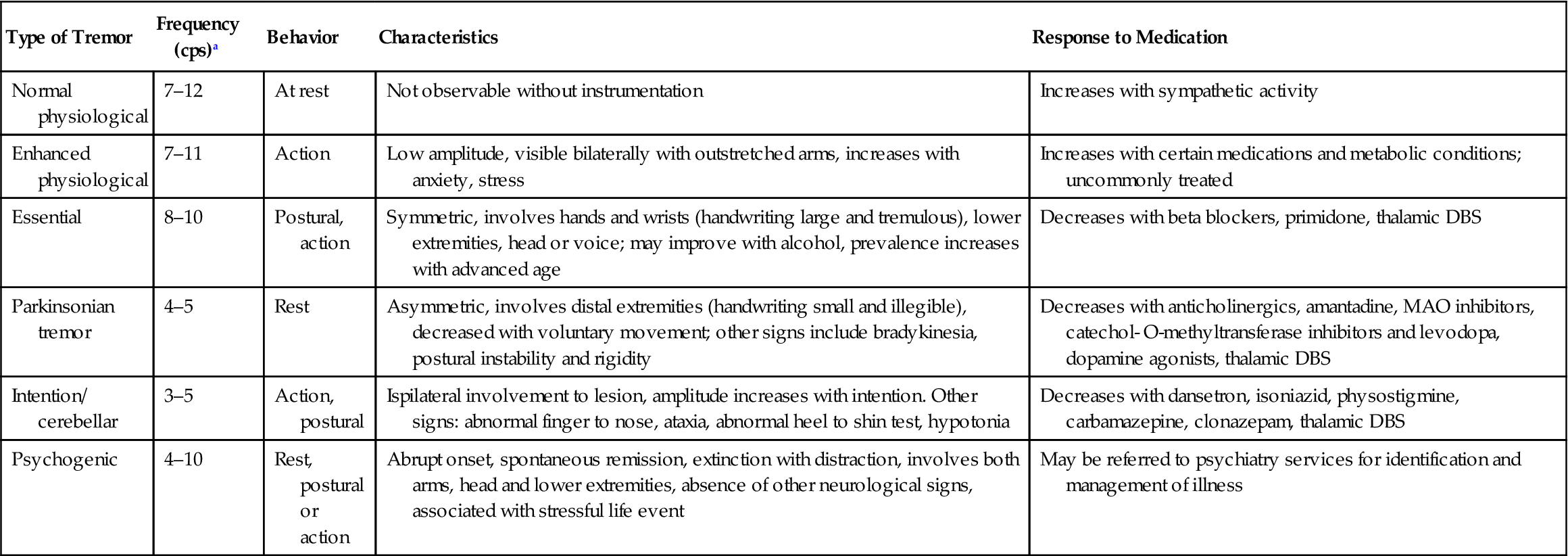
aFrequency range: low 0–4, middle 4–7, high 7–12 cps (cycles per second). Monoamine oxidase (inhibitor), MAO
Sources: Alty and Kempster, 2011; Bhidayasiri, 2005; Daroff et al., 2012; Deuschl et al., 1998; Klein, 2005.
Because the frequency (period) of most tremors is remarkably stable within and across individuals, one classification strategy focuses on the frequency of the tremor as it typically occurs. This requires electromyographic (EMG) recording or use of a sensitive accelerometer; tremor frequency cannot be reliably assessed by observation alone. Amplitude of tremor is more variable, both within and among individuals (e.g. becoming more pronounced under stressful conditions or with fatigue), and therefore is not a useful indicator of severity of tremor.
A more common way to classify tremor is based on when the tremor is observed. A resting tremor occurs in an otherwise relaxed or inactive body part. Resting tremors are commonly observed in individuals with Parkinson’s disease (e.g. ‘pill-rolling’ tremor of the hands), as described in Chapter 30, and may also be seen in those with normal pressure hydrocephalus, heavy metal poisoning and neurosyphilis, or as a side-effect of the use of neuroleptic medications. A postural tremor occurs when a body part (limb or trunk) is maintained in a sustained, often antigravity, position. Postural tremor is frequently a component of essential tremor and may also be observed in Parkinson’s disease, hereditary motor and sensory neuropathy (Charcot–Marie–Tooth disease) and spastic torticollis. An action tremor (kinetic tremor) occurs during volitional movement. In those with essential tremor, the amplitude of an action tremor remains stable throughout the excursion or performance of the movement. Action tremors that worsen (increase in amplitude) during the trajectory of the movement, especially as the movement goal is approached, are referred to as intention tremors. Intention tremors are clinically evaluated using ‘finger-to-nose’ or ‘heel-to-shin’ movement tasks. Intention tremors are classic signs of cerebellar dysfunction.
Neurologists often evaluate the response to medication as a means of confirming or clarifying the diagnosis of a movement disorder.
The amplitude of resting tremors often decreases when anticholinergic medications are administered. The amplitude of essential tremors (whether action or postural) tends to diminish with consumption of alcohol or administration of beta blockers. Cerebellar intention tremors are unresponsive to pharmacological intervention and intensify with alcohol consumption (Rowland & Pedley, 2010; Alty & Kempster, 2011; Crawford & Zimmerman, 2011).
Physiological tremor
Physiological tremor is a normal phenomenon that is usually so mild that it cannot be easily observed at rest (Whitney et al., 2003). A fine physiological tremor of 11–13 cycles per second (cps) can be detected in healthy individuals on EMG; this is usually not observable without instrumentation. Because this minimal amplitude physiological tremor is normal in all muscles of the body, it is observed during movement and while holding antigravity positions. Factors that contribute to physiological tremor include the resonant properties of musculoskeletal structures; synchronization of agonist/antagonist motor neuron activity coupled by afferent neurons from the muscle spindle; and the cardioballistic force of the heartbeat. Physiological tremor affects all muscles of the body simultaneously whereas most pathological tremors tend to affect selected body segments. Physiological tremor may become ‘enhanced’ with any mechanism that triggers sympathetic nervous system activity (beta-adrenergic activity and catecholamine release), including stress, anxiety, fright, sleep deprivation, alcohol ingestion, certain classes of cardiac medication, CNS stimulants, exercise and fatigue. The amplitude of physiological tremor also increases in hypoglycemia, thyrotoxicosis, alcohol and sedative withdrawal, carbon monoxide exposure and heavy metal poisoning. Toxic levels of certain medications (lithium, bronchodilators, tricyclic antidepressants) may also lead to tremor. Physiological tremor typically becomes more difficult to detect with advancing age.
Essential tremor
Essential tremor can be observed as a postural and/or action tremor, commonly affecting neck and axial muscles, expressed as a nodding rotation of the head or an oscillating flexion/extension movement of the trunk (Sullivan et al., 2004). It may be apparent during upper extremity tasks that require holding a fixed proximal position. Involvement of the muscles of the larynx and pharynx may compromise phonation and swallowing. As an action tremor, essential tremor may interfere with the efficiency of fine motor tasks such as writing, grooming or bringing food on utensils toward the mouth. A recent meta-analysis has indicated the prevalence of essential tremor to be between 0.01% and 20.5% of the population. The prevalence in individuals aged 65 years or older is 4.6% and may be as high as 21.7% in those 95 or older (Louis & Ferreira, 2010).
Essential tremor, although considered benign because it is not associated with progressive neuropathology, is generally bilateral and can significantly interfere with functional activities in older adults. There is often a temporary decrease in symptoms (for approximately 30 minutes) after ingestion of alcohol (Mostile & Jankovic, 2010). Propranolol and other beta-blocker medications are prescribed for long-term management when essential tremor interferes with function, except when contraindicated by other concurrent conditions (e.g. congestive heart failure, atrioventricular (AV) heart block, asthma, insulin-dependent diabetes). Primidone (Mysoline), an anticonvulsant, may also be prescribed. Sedatives, tranquilizers and anticholinergics have little impact on essential tremor.
Thalamic deep brain stimulation (DBS), a procedure that uses implanted pulse generators in the subthalamic nucleus or globus pallidus, has been performed in individuals with essential tremor. Reports have indicated a reduction in contralateral tremor by as much as 75% in up to 90% of the cases (Pahwa et al., 2006). This procedure is only considered in healthy patients who are cognitively intact and when the tremor is resistant to medications. Side-effects are rare but may include neurological implications such as an intracranial hematoma, headaches, dyspraxia and word-finding difficulties (Daroff et al., 2012).
Resting tremor
Resting tremor is a tremor at rest that disappears with volitional movement. Resting tremor is one of the most common symptoms of Parkinson’s disease and may also be seen in other neurological conditions such as normal pressure hydrocephalus, progressive supranuclear palsy and the cumulative encephalopathy in those with repetitive head injury (Krauss & Jankovic, 2002; Jankovic & Tolosa, 2007). It most often involves oscillating supination/pronation of the forearm or lumbrical flexion/extension of the thumb and fingers (e.g. ‘pill-rolling’ tremor). Parkinsonian resting tremor has a relatively low period/frequency when compared with other types of tremor. Although the underlying mechanism is unclear, it may be the result of compromised nigral–striatal function. Anticholinergic medications (e.g. trihexyphenidyl/Artane, benzatropine/Cogentin) are more effective in reducing resting tremor than dopamine agonists or levodopa. Surgical ablation of the contralateral ventral lateral (VL) nucleus of the thalamus has been used to reduce the amplitude of severe resting tremor.
Intention tremor
An intention tremor is a tremor that becomes obvious and often exaggerated as the need for precise movement increases (also known as rubral, cerebellar or ‘course’ tremor) (O’Suilleabhain & Dewey, 2004; Weiner & Lang, 2005). With intention tremor, there is oscillation of increasing amplitude during voluntary movement, especially as the movement draws to its conclusion. Intention tremor is one of the symptoms of cerebellar dysfunction, especially if there has been damage to the superior cerebellar peduncle because of diffuse axonal injury, multiple sclerosis or infarction/ischemia in the midbrain and upper pons. Because damage to these structures compromises the ongoing ‘feedback’ necessary for ‘error control’, intention tremor is most apparent when fine-skilled motor tasks are attempted. In addition, intention tremor has been observed in alcohol, barbiturate or sedative intoxication and with high serum levels of some anticonvulsants (e.g. phenytoin/Dilantin and carbamazepine/Tegretol).
Intention tremor affects proximal and distal musculature of the extremities. In very severe cases, there may be observable postural tremor in addition to the classic disruption of goal-oriented volitional movement. Individuals with intention tremor may also exhibit other symptoms of cerebellar dysfunction including nystagmus, hypotonia, dysmetria, movement decomposition and gait ataxia. For reasons not well understood, the amplitude of cerebellar intention tremor often decreases when the eyes are closed.
Neuropathic tremor
Occasionally, tremor has also been observed in individuals with significant peripheral neuropathy; however, the presentation of neuropathic tremor is much less stereotypical than essential, resting and intention tremors (Daroff et al., 2012). It is not well understood how and why tremor occurs in individuals with neuropathy.
Neuropathic tremor occurs in some, but not all, individuals with longstanding diabetes, end-stage renal disease, chronic alcoholism, hereditary sensory–motor neuropathy (Charcot–Marie–Tooth disease) and infectious neuropathies such as acute Guillain–Barré syndrome. These tremors may present as action and resting tremors based on whether it is of demyelinating or inherited origin. Management of these tremors can be challenging because many of the medications that are successful in controlling extraneous movement are not as effective in the presence of peripheral neuropathy (Puschmann & Wszolek, 2011). These disorders need to be diagnosed in a timely manner as they can be treated with immuunosuppressive therapies, such as corticosteroids, intravenous immunoglobulin (IVIG), cyclophosphamide or plasma exchange.
Post-Traumatic tremor
Individuals of any age who have sustained a severe acquired brain injury may develop a tremor within a month or years after the actual trauma and can have a mixed presentation. The tremor most commonly manifests itself 1–4 weeks after the traumatic event, and it is similar to essential tremor (Krauss & Jankovic, 2002; O’Suilleabhain & Dewey, 2004). A delayed-onset post-traumatic tremor, evolving 12–18 months post injury, has also been reported. Delayed-onset post-traumatic tremor often persists for several years or longer. It is not possible to identify a specific lesion eliciting the tremor by magnetic resonance imaging (MRI) or computed tomography (CT) scanning. This type of tremor is not particularly responsive to the medications used to control essential tremor. Often, the magnitude of this post-traumatic tremor decreases over time; however, it may remain problematic for some individuals.
Orthostatic tremor
In very rare circumstances, an older adult may experience a tremor in their lower extremities only during unsupported standing or during preparation for assuming a standing position. If the tremor is severe, it can interfere with transitional movement (e.g. sit-to-stand) and with postural control (Whitney et al., 2003). Orthostatic tremor is usually perceived by the individual as difficulty with stability (unsteadiness) while standing, when not supported by an assistive device or other external support, and is frequently associated with an increased fear of falling. Orthostatic tremor has a higher frequency/faster period (14–18 cps) than most other tremors, although its amplitude tends to be small. Orthostatic tremor does not respond to alcohol or propranolol, as seen in essential tremor, but does respond to clonazepam. Levodopa and gabapentin have also been recently used to treat orthostatic tremor and beneficial results have been noted (Daroff et al., 2012). Orthostatic tremor can significantly affect quality of life and limit functional ability.
Metabolic tremor
Metabolic tremor is characterized by a recent-onset postural tremor. Hyperthyroidism is the most common cause of metabolic tremor and presents as a high frequency tremor of the upper extremities that is similar to an enhanced physiological tremor as seen with anxiety or stressful triggers. Additional systemic signs accompany the tremor, such as excessive sweating or weight loss, which will differentiate it from an essential tremor. Other metabolic causes include renal failure, hypoglycemia and liver disease (Alty & Kempster, 2011).
Psychogenic tremor
Psychogenic tremor is a psychiatric condition that appears as involuntary movement, differing in characteristics and consistency from action, intention or resting tremors (Alty & Kempster, 2011). Within an individual, psychogenic tremor may migrate from one area of the body to another. Onset is typically abrupt and may occur after a stressful life event; most other types of tremor are insidious. The frequency and amplitude of psychogenic tremor are inconsistent and variable over time. In most other types of tremor, the amplitude tends to increase when individuals are given competitive, anxiety-producing cognitive tasks (e.g. beginning at 100 and serially subtracting 7); in psychogenic tremor, the amplitude tends to decrease (or completely disappear) when attention is focused elsewhere.
Holmes’ tremor
Holmes’ tremor is an uncommon tremor caused by damage to the cerebellar connections to the thalamus and brainstem (Seidel et al., 2009). Holmes’ tremor was previously referred to as rubral tremor, midbrain tremor, thalamic tremor and Benedikt’s syndrome. This tremor is a postural tremor of the proximal limbs characterized by slow frequency and large oscillations. It can be present at rest, during postural control (sitting) and may increase with movement. Multiple sclerosis and brain injury are common causes for Holmes’ tremor (Alty & Kempster, 2011).
Classification and differential diagnosis of dyskinetic conditions
The other types of dyskinetic conditions that are encountered in geriatric rehabilitation are most often associated with a long-term disorder with which the individual has aged; however, some medication-related movement disorders are newly diagnosed.
Huntington’S chorea
Huntington’s chorea is an autosomal dominant hereditary progressive disorder involving degeneration of the corpus striatum (Rowland & Pedley, 2010; Daroff et al., 2012). The gene for Huntington’s is located on the short arm of chromosome 4. The three characteristic signs of Huntington’s chorea are movement disorder (chorea), dementia and personality disorder. The first signs and symptoms of the disease appear in midlife (35–40 years) as restlessness, emotional lability, neurosis or personality disorders. Over time, cognitive impairment becomes more apparent and choreiform involuntary movement develops, often impairing judgment, locomotion and mobility, speech production and swallowing. As the severity of symptoms increases, functional status deteriorates. Dystonia and rigidity may develop late in the disease process.
On CT or MRI there is marked bilateral degeneration of the caudate nucleus, enlargement of the anterior horn of the lateral ventricles and cerebral atrophy. Treatment of Huntington’s disease is symptomatic; choreiform movement can sometimes be managed with dopamine-blocking agents such as haloperidol, reserpine or tetrabenazine. Individuals with Huntington’s disease may have to cope with their increasingly debilitating impairments for 10–25 years, until the disease takes their life.
Wilson’S disease (hepatolenticular degeneration)
Wilson’s disease (WD) is a rare autosomal recessive hereditary disorder of copper metabolism, with a mutation on chromosome 13 (Rowland & Pedley, 2010; Daroff et al., 2012). Many patients present in childhood with signs of liver disease and failure associated with the accumulation of copper. Although initial symptoms typically appear in adolescence and early adulthood, presentation may first occur as late as 60 years of age. If undetected early in life, Wilson’s disease can be fatal.
Nearly half of patients with WD present with CNS signs and symptoms (Daroff et al., 2012). Neurological symptoms of poorly managed WD include resting or postural tremor, chorea of the extremities, dystonia, pseudobulbar palsy and cognitive dysfunction. The abnormal liver function associated with WD eventually leads to chronic cirrhosis. Traditionally, the acute management of the disease began with penicillamine, but more recent treatment strategies include the use of trientine and zinc (removes excess copper) or ammonium tetrathiomolybdate (to block copper absorption), which are less toxic but still being researched. Orthotopic liver transplantation has been found to correct the underlying pathology in those individuals in severe hepatic failure without the presence of neurological symptoms (Daroff et al., 2012).
Paroxysmal kinesigenic dyskinesia
Paroxysmal kinesigenic dyskinesia (PKD or paroxysmal kinesigenic epilepsy), formerly known as paroxysmal choreoathetosis, presents as jerking and writhing movements of the limb and trunk when an individual is unexpectedly startled or disturbed (Daroff et al., 2012). The movements may be unilateral or bilateral lasting about 1 minute and can occur up to 100 times daily. Paroxysmal dyskinesia initiates in childhood, however this condition persists later in life. PKD is responsive to anticonvulsant medication such as carbamazepine and phenytoin.
Paroxysmal nonkinesigenic dyskinesia
Paroxysmal nonkinesigenic dyskinesia (PNKD), formerly known as familial choreoathetosis, is a rare autosomal dominant hereditary movement disorder that is relatively benign, with onset in childhood or early teens (Rowland & Pedley, 2010). In this condition, the individual experiences intermittent ‘attacks’ or spells of dystonia, chorea, athetosis, ballismus or a combination of movements that are associated with periods of physical exertion or ingestion of alcohol or caffeine. The ‘attack’ frequency can range from several episodes per month to several episodes per day, and last between 10 minutes to several hours (Daroff et al., 2012). This condition may lessen with age.
Senile chorea
Senile (or essential) chorea is a late-appearing idiopathic movement disorder that evolves in the absence of psychotropic or dopamine therapy, Huntington’s chorea, dementia or familial movement disorders (Daroff et al., 2012). Also known as oral–facial–lingual dyskinesia, senile chorea primarily affects the muscles of the mouth, tongue and jaw. It is important to differentiate the abnormal involuntary movements of senile chorea from the similar facial movements that occur in tardive dyskinesia and the lip and jaw movements commonly observed in older individuals who have lost all of their teeth and are no longer able to wear dentures. Antidopaminergic drugs are the most effective when monitored carefully to assess for the development of tardive dyskinesia.
Tourette’S syndrome
Tourette’s syndrome is a genetically determined chronic neuropsychiatric disorder that is characterized by multiple motor and vocal tics (Hallett, 2003; Daroff et al., 2012). Symptoms may occur initially in childhood or adolescence and persist into adulthood and later life. Initially, many individuals with Tourette’s are misdiagnosed with a psychiatric illness.
Initial motor tics typically involve the face and eyes, and may eventually include vocalizations (repetitive grunts, barks, throat clearing, cursing, echolalia). Repetitive motor tics of the extremities can resemble chorea. Although Tourette’s is documented as an autosomal dominant trait, other hypotheses are: it is considered to be a disorder of the basal ganglia that involves excessive levels of the transmitter dopamine; a limbic system disorder involving dysfunction of the central endogenous opioid system; and reports of Tourette’s following head trauma, toxic and metabolic encephalopathies, and Huntington’s disease are more likely to be coincidental than causal.
Tourette’s can cause considerable social, vocational and functional impairment and may also be associated with obsessive–compulsive behavior, attentional and executive dysfunction, sleep disorders and aggressive behavior (Hankey & Wiardlaw, 2008). Lifelong pharmacological intervention including dopamine-blocking agents, clonidine, haloperidol or pimozide can assist with community reintegration without compromising life expectancy.
Drug-Induced movement disorders
Extrapyramidal dysfunction also occurs as the undesirable side-effect of antipsychotic drugs and other medications (Caligiuri et al., 2000; Lee et al., 2005; Morgan & Sethi, 2005). Because drug metabolism and excretion mechanisms become less efficient with aging, older adults are more susceptible to drug toxicity and adverse drug reactions; the medications that older adults are prescribed may remain physiologically active for longer periods of time, especially if dosage is not adjusted for age and body composition (Ciccone, 2007; Gladson, 2010). Classes of medications associated with extrapyramidal side-effects are outlined in Table 31.2.
Table 31.2
Medications associated with extrapyramidal side-effects
| Type of Medication | Symptoms | Examples |
| Antipsychotic/neuroleptic | Akathisia, pseudo-Parkinson’s chorea, tardive dyskinesia, acute dyskinetic reaction | Traditional antipsychotics: chlorpromazine, triflupromaxine, fluphenazine, perphenazine, trifluoperazine, promazine, mesoridazine, thiothizene, haloperidol, loxapine, molindone Atypical antipsychotics: aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone |
| Antidepressants | Chorea, athetosis, akathisia Tremor, myoclonus, pseudo-Parkinson’s chorea | Tricyclic antidepressants, mono-oxide inhibitors Lithium carbonate, amoxapine |
| Stimulants | Postural tremor, chorea | Amphetamines, methadone, methylphenidate, fenfluramine, caffeine, cocaine |
| CNS depressants/sedatives | Physiological intention tremor, chorea, dystonia | Alcohol, diazepam |
| Anticonvulsants | Intention tremor, chorea, asterixis | Phenytoin, valproic acid, carbamazepine, phenobarbital, clonazepam |
| Anti-Parkinson’s medications | Akathisia, chorea, dystonia | Amantadine, bromocriptine, levodopa |
| Other types of medication | Tremor | Bronchodilators (theophylline, doxapram), hypoglycemics, corticosteroids |
| Chorea, tremor | Gastrointestinal medications (cimetidine, terfenadine) | |
| Chorea, dystonia, tremor | Antiarrhythmic medications (propranolol, tocainide) | |
| Tardive dyskinesia | Antiemetic medications (prochlorperazine, thiethylperazine, promethazine) | |
| Intention tremor, ataxia | Cyclosporin A | |
| Chorea | Estrogen/oral contraceptives, sleeping medications |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







