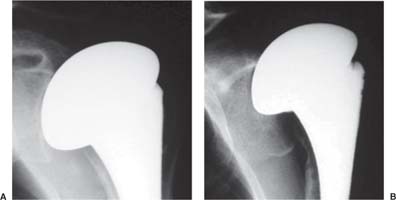
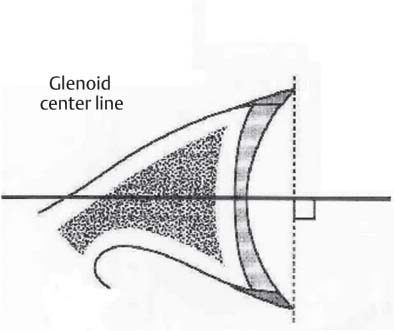
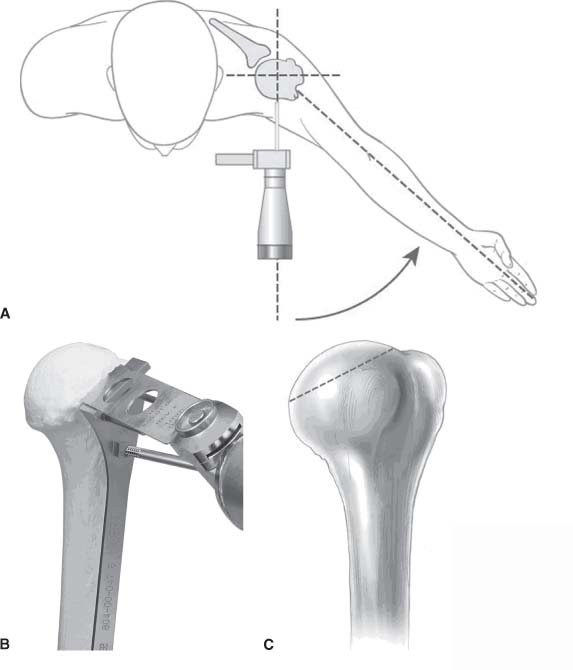
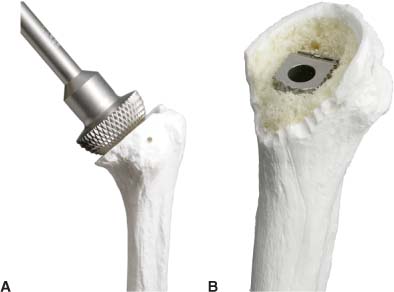
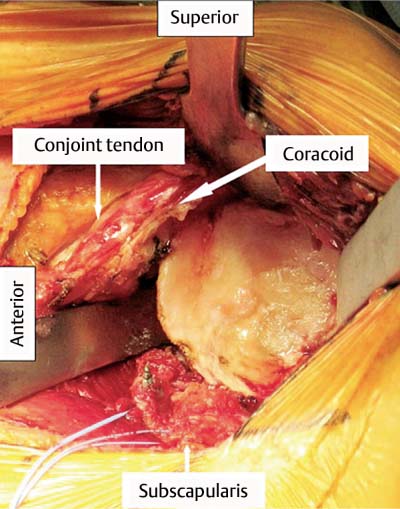
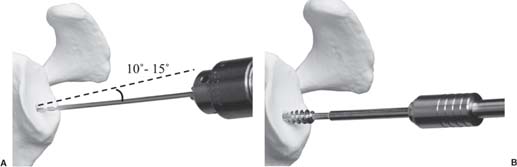
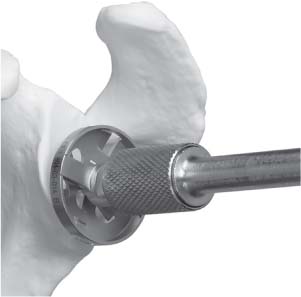
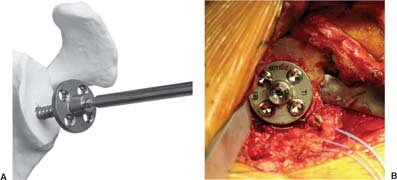
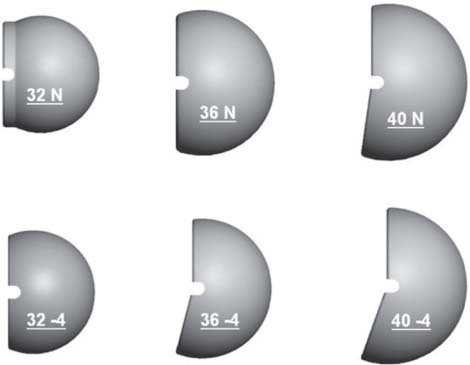
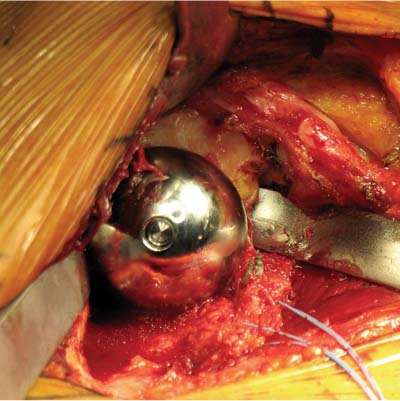
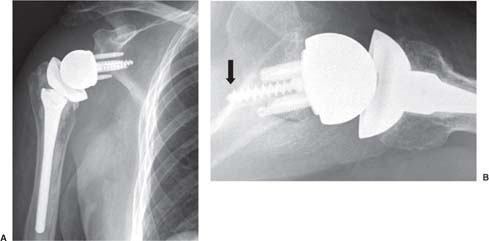
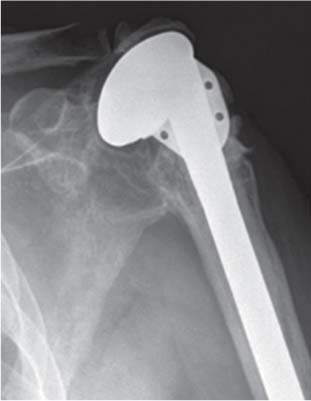
13 Treating the Rotator Cuff–Deficient Shoulder: The Florida Orthopaedic Institute Experience Based in Tampa, Florida, the Florida Orthopaedic Institute is a multispecialty orthopedic group that was formed in 1990. This practice has evolved over the past 16 years and is currently composed of 30 orthopedic surgeons who are all subspecialty trained and practicing in the various disciplines of orthopedic surgery. Each year the group routinely sees over 150,000 patient visits and performs over 12,000 surgical procedures. In 1992, the Shoulder and Elbow Service of the Florida Orthopaedic Institute was formed. Since its inception, the service has rapidly grown and now treats a large number of patients with a wide range of shoulder pathology. Last year, the service had 13,000 patient visits, and a substantial number of these patients, ~2,200 suffered from a combination of arthritis and rotator cuff (RC) dysfunction. This extensive clinical experience with the RC-deficient shoulder since the early 1990s has led to an evolution in the way we evaluate and care for these patients. The manner in which we surgically treat these patients has markedly changed over the years in an effort to improve patient function and outcome. I will attempt to present our experiences and the knowledge we have gained in our effort to treat the RCdeficient shoulder. In the early 1990s, the treatment of the RC-deficit shoulder remained a dilemma. Multiple treatments had been tried over the course of several decades in an effort provide pain relief and improved function in this group of patients with irreparable cuff tears. In the RC-deficient shoulder, the force couple of the joint is disrupted, allowing the deltoid to produce a change in the overall direction of the joint forces and destabilize the glenohumeral joint with its superiorly directed pull.1 In the 1970s, surgeons attempted to offset this through glenohumeral arthrodesis; however, they found this procedure ineffective. Cofield and Briggs2 reported on their arthrodesis experience of 12 patients with RC-tear arthropathy in 1979. Six of the 12 required another operation due to proximal migration, nonunion, or acromioclavicular pain. Surgeons also attempted to use constrained total shoulder arthroplasty in the early 1970s and 1980s in an effort to treat the RC-deficient shoulder. Due to the constraints of this prosthesis, these implants were found to have a significant failure rate with early glenoid loosening. In 1982, Lettin et al3 reported on 10 of 49 shoulders who were treated in this manner and developed early glenoid component loosening; eventually, most surgeons deserted this total shoulder construct. The use of unconstrained total shoulder arthroplasty was also tried in an attempt to treat this difficult problem. This method was found to be problematic secondary to the “rocking horse” effect on the glenoid as described by Franklin and colleagues.4 They found that superior migration of the humeral head correlated with increased glenoid loosening due to the eccentric forces the glenoid was experiencing. Subsequently, unconstrained arthroplasty fell out of favor. At the start of my practice in the early 1990s, hemiarthroplasty had become the gold standard of treatment in the RC-deficient shoulder. Multiple studies5–7 reported that this approach produced significant pain relief with variable gains in forward elevation. Successful results were reported at 60 to 80% using Neer’s limited goals criteria. However, some surgeons, including myself, were not fully satisfied with the results of this procedure. Sanchez- Sotelo6 and colleagues reported on 33 shoulders in 30 patients who had undergone a hemiarthroplasty for cuff tear arthropathy (CTA) and found that progressive superior migration and progressive glenoid bone loss occurred in 8 of 33 shoulders and 16 of 33 showed acromial bone loss with two fractures. I too noted that many of my patients had initial pain relief with this operation, but with longer follow-up demonstrated progressive anterosuperior instability, progressive bone loss, and unsatisfactory results. We decided to review retrospectively 21 consecutive hemiarthroplasties we performed for CTA, which had at least 2 years of follow-up. The average age of these patients was 71.5 years old and the mean follow-up was 73 months. Five of the 21 patients had previous RC surgery. All of these patients underwent an anatomical humeral head replacement. In this group of patients, we saw a decrease in their level of pain, but no significant improvement in function. The postoperative ASES and VAS function, forward flexion, and abduction did not improve significantly from the preoperative values. With respect to patient satisfaction, only 24% of the responses were excellent or good after hemiarthroplasty. In fact, 47% of the patients were dissatisfied after hemiarthroplasty. The radiographic findings in this study were equally discouraging. Sixteen of these 21 patients demonstrated progression of proximal subluxation or progression of erosion of the glenoid (Fig. 13–1). The location of progressive instability and erosion was most commonly superior and anterior. Many of these hemiarthroplasties were deemed failures and the reoperation rate in this series was 35%. Given these results I began to search for a better way to treat these patients. Figure 13–1 (A,B) A postoperative x-ray of a hemiarthroplasty preformed for rotator cuff deficiency. Note the proximal migration of the humeral component and the erosion of the superior aspect of the glenoid. From experience, I determined that hemiarthroplasty was not solving the problem of the RC-deficient shoulder. I became interested in a surgical procedure that was being performed in Europe: surgeons were using a “reversed” shoulder design that had been tried almost three decades ago.7 The initial attempts had used a small glenoid ball within a large humeral socket; this had resulted in a highly constrained arthroplasty. This ultimately had led to high rates of glenoid loosening and hence the procedure was abandoned. In the early 1990s, Grammont and Baulot8 reported on the Delta (Depuy Orthopaedics, Inc., Warsaw, Indiana) reversed shoulder arthroplasty for CTA. In their design, they used a smaller humeral socket in relation to a larger glenoid hemisphere with the center of rotation (COR) at the glenoid. This design resulted in pain reduction and improvement in functional ROM in multiple studies.9,10 To learn more about the reverse design and procedure, I traveled to Europe and spent time with Gilles Walch (Clinique Sainte Anne Lumière, Lyon, France) and Pascal Boileau (Hôpital de l’Archet, Nice, France), from whom I learned a great deal about the Delta (Depuy, Warsaw, Indiana) prosthesis. I was interested in using this prosthesis for one of my patients; however, I could not get Food & Drug Administration (FDA) approval to use the device in the United States. So, I began designing a reverse prosthesis in effort to treat the cuff-deficient shoulder. I sought to keep the basic concept of Grammont’s reversal of the anatomy, but I wanted the COR to remain outside of the glenoid as it is in the normal shoulder anatomy. This led to my design of the Reverse Shoulder Prosthesis (RSP; Encore Medical Corp., Austin, Texas) (Fig. 13–2). Figure 13–2 The Reverse Shoulder Prosthesis. From 1998 to December 2007, I had performed 773 reverse shoulder arthroplasties. We have kept a database on these patients since 1999 to promote our understanding of how this device treats the RC-deficient shoulder. We evaluate several outcome measures on each of our patients preoperatively and postoperatively and videotape each patient to assist in documenting the ROM. This has allowed us to obtain a wealth of information on this patient population. Currently, my indications for the use of a RSP are -Irreparable RC tear with glenohumeral arthritis -Irreparable RC tear with glenohumeral instability -Failed hemiarthroplasty – Painful and loose total shoulder arthroplasty with RC deficiency My contraindications for this surgical procedure are -Nonfunctional deltoid muscle -Active sepsis -Excessive glenoid bone loss -Debilitating neurologic disorder -Metal allergy Patients who meet the above indications and have failed conservative management are candidates to undergo the following surgical techniques. All patients who undergo a primary RSP receive the same preoperative workup.11 All patients must have recent x-rays and a preoperative computed tomography (CT) scan. The x-rays show the position of the humerus relative to the glenoid and reveal the degenerative changes of the humerus, glenoid, and acromion. The axial cuts of the CT scan are evaluated to look at the wear pattern on the glenoid and plan for proper central screw placement of the baseplate. In patients who demonstrate minimal glenoid bone loss, the ideal position of the central screw will follow the path of the centering line as described by Matsen and Lippitt12 (Fig. 13–3), in which the central screw exits anteriorly on the scapular neck. This typically will provide at least 25 mm of bone for the screw to achieve purchase. In the majority of our primary RSP cases, we are able to position the central screw in this position. For surgery, the patient is placed in the upright beach-chair position with the head firmly secured and the arm draped free. The operative arm is positioned sufficiently off the side of the table to allow for unobstructed movement in adduction and hyperextension of the shoulder (Fig. 13–4). The patient is administered a general anesthetic in addition to a scalene block. An extended deltopectoral approach is employed and up to two thirds of the pectoralis major tendon is released. The subdeltoid, subacromial, and subcoracoid spaces are released. If the subscapularis tendon is intact, it is released off the lesser tuberosity just medial to the long head of the biceps, allowing atraumatic dislocation of the humeral head with gentle external rotation (ER) and extension of the arm. The capsule is then released completely around the humeral neck. Aggressive resection of any osteophytes is then performed. A neck cut is made in 30 degrees of retroversion. This cut is made at a slightly higher level than for a traditional arthroplasty (Fig. 13–5). Sequential broaches are used to prepare the canal. Figure 13–3 Ideal position of the central baseplate screw. This screw follows the centering line as described by Matsen.12 Figure 13–4 Patient prepped and draped in the beach-chair position. Figure 13–5 (A) The humerus is cut in 30 degrees of retroversion using a version guide and the forearm as a reference. (B) Cutting guide is placed; an oscillating saw is used for the humeral head cut. (C) A thin head cut is used for Reverse Shoulder Prosthesis cases. Figure 13–6 (A,B) Proximal humeral reamers are used to prepare the humerus. The proximal humerus is then reamed using the smallest metaphyseal reamer (Fig. 13–6A). Any remaining osteophytes and a portion of the calcar are then resected back to a recessed position (Fig. 13–6B). The humeral broach is left in place until implantation of the glenoid component is completed. This sequence allows for sufficient resection of the proximal humerus to aid in glenoid exposure. The delay of the last two humeral reamers until the glenoid preparation is complete maintains adequate humeral bone stock to support retraction during glenoid preparation. Figure 13–7 A 360-degree periglenoid exposure is performed to prepare for baseplate insertion. Glenoid exposure is accomplished by retracting the proximal humerus posteriorly utilizing a posterior glenoid retractor, and performing an aggressive 360-degree subperiosteal periglenoid capsular release. A Hohman retractor is then placed anteriorly on the glenoid neck, and a second Hohman retractor is placed at the superior aspect of the glenoid (Fig. 13–7). With protection of the axillary nerve, the inferior capsule is then resected. Once satisfactory visualization of the glenoid is accomplished, a centering hole is drilled using a 2.0-mm drill with a slight inferior tilt, followed by the 6.5-mm tap (Fig. 13–8). The tap is left in the glenoid to serve as a guide for placement of the cannulated glenoid reamers. Sequential cannulated convex ream-ers are then used to prepare the glenoid for the baseplate insertion (Fig. 13–9). Next, a fixed angle hydroxyapatite-coated glenoid baseplate is screwed into place with secure purchase (Fig. 13–10). Four 5.0-mm locking peripheral fixation screws are inserted into the glenoid baseplate. In cases where the locked screw pathway does not have sufficient bone, 3.5-mm nonlocking cortical screw is used and angled to achieve secure fixation in bone. An appropriately sized glenosphere (32-mm neutral, 32 – 4 mm, 36-mm neutral, 36 – 4 mm, 40-mm neutral, 40 – 4 mm) (Fig. 13–11) is then selected, depending on the degree of soft tissue contracture, the size of the patient, the quality of glenoid bone, and the expected degree of instability. It is placed onto the baseplate via a Morse taper. A retaining screw is then placed into the central hole on the glenosphere to augment the Morse taper attachment to the baseplate (Fig. 13–12). The humeral reaming is then completed and a trial humeral socket is chosen from a selection of sizes (neutral, neutral-semi-constrained, +4 mm, +4-mm semi-constrained, +8 mm, +8-mm semi-constrained) depending on the soft tissue balancing and degree of instability. After reduction with the humeral broach and a trial humeral socket (Fig. 13–13), transosseous sutures are placed into the lesser tuberosity for future subscapularis repair. Next, the appropriate-size humeral implant that would allow a 2-mm circumferential cement interface around the component is selected and routinely cemented in place with antibiotic-laden cement. Our standard practice has been to use antibiotic-laden cement in all cemented arthroplasties as it has been found to reduce the risk of deep wound infection. The joint is then reduced and checked for stability especially in abduction, extension, and internal rotation (the position of greatest instability) and achievement of full passive elevation is confirmed. Finally the subscapularis is repaired through drill holes followed by routine closure using #2 braided polyester sutures. Standard radiographs are obtained immediately postoperatively (Fig. 13–14). Figure 13–8 (A) 2.5-mm drill is oriented with 10 to 15 degrees of inferior tilt. (B) The glenoid is tapped along the drill path. Figure 13–9 The glenoid is reamed over the tap. Figure 13–10 (A) The glenoid baseplate is then inserted. (B) Implanted Reverse Shoulder Prosthesis baseplate with four 5.0-mm locking screws. A shoulder immobilizer is worn for 6 weeks while pendulum-type exercises are performed. After the first 6 weeks, the patient is transitioned to a sling and supine active assisted ROM exercises are initiated. Active assisted elevation can begin at 6 weeks, but resistive exercises are delayed until 12 weeks after surgery. Strengthening and stretching exercises should continue with maximal functional improvement expected to occur about one year after surgery. Figure 13–11 There are several options for glenosphere selection with varying diameters and offsets. Figure 13–12 An implanted glenosphere. In my practice, I encounter a high volume of referred patients who have had previous arthroplasty and remain symptomatic with a RC that is nonfunctional. These patients present with pain and poor function of the affected extremity. Their previous arthroplasties have included hemiarthroplasty or a bipolar, unconstrained total shoulder arthroplasty, and reverse shoulder arthroplasty. The etiologies for the initial arthroplasty have included proximal humeral fracture, CTA, and glenohumeral arthritis. Figure 13–13 A sawbones model demonstrating a reduction with trial components in place. Figure 13–14 (A,B) Postoperative x-ray of the Reverse Shoulder Prosthesis. In these groups of revision cases, special technical measures are required. Each of these groups of patients has a specific pathology that can be due to the previous procedure they have undergone. We classify these patients into different groups based on their prior procedure because this tends to dictate the type of pathology the patient will have during revision surgery. This allows us to have a preoperative plan as to what obstacles may be encountered during the revision and enables us to have all the necessary equipment present and ready for the case. Therefore, one must have an effective way to remove the previous implant, and then deal with the additional pathology that can be present in each of these groups of patients. For instance, when revising a hemiarthroplasty for fracture, the surgeon should be prepared to deal with scarred down tuberosities, the proximal humeral bone loss and instability that can be present after implant removal. To optimize results, we attempt to recognize this in the pre-operative setting and plan for it. Additionally, a bipolar or a hemiarthroplasty with a larger humeral head can produce a patulous deltoid, making stability an issue in attempting to convert to the RSP. In this situation, one must anticipate the need for more conforming sockets and replacing bone loss to avoid postoperative dislocation. In cases of revision after hemiarthroplasty for CTA, one must be prepared for the glenoid bone erosion that often has occurred and may require bone grafting. If it is an unconstrained total shoulder that is being converted, the surgeon should have an effective way of removing the glenoid component and cement to prepare a good bony bed for baseplate implantation. Lastly, in revising a reverse shoulder arthroplasty the surgeon must be prepared to encounter broken screws and other failed hardware. The following sections illustrate our current surgical approach of converting to the RSP in these complex cases, and have provided us with reasonable outcomes, which are described below. The primary concern in the revision surgery of a hemiarthroplasty for fracture is removal of the implant and the potential for proximal humeral bone loss as well as instability (Fig. 13–15). We obtain recent plain x-rays and a CT scan of these patients prior to surgery. The plain films give us information regarding what type of humeral component is in place so we can plan for its removal, and also allows us to look at the tuberosities. The CT scan is imperative to evaluate proximal humeral bone stock as well as the position of the tuberosities because they are commonly in a malunited or nonunited position. If the CT scan reveals a greater tuberosity that has retracted to a posterior position, it is valuable information in that we will have to look for this fragment at the time of surgery. Failure to remove this fragment at the time surgery can influence the patient outcome and cause pain, a decrease in postoperative ROM, or possible instability. The CT scan also provides detail of the glenoid anatomy to plan for the central screw placement of our baseplate. Before these cases, we are sure to request a proximal humeral allograft as this may be required during surgery. Figure 13–15 Failed hemiarthroplasty with proximal humeral bone loss, superior migration of the humeral component, and glenoid erosion.
Early Experience
Reversing the Trend
Using the Reverse Shoulder Prosthesis
Preoperative Considerations and Operative Technique
Primary Reverse Shoulder Prosthesis
Postoperative Rehabilitation
Previous Arthroplasty and Conversion to Reverse Shoulder Prosthesis
The Problems of Instability and Bone Loss
Conversion Hemiarthroplasty for Fracture
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree