Fig. 18.1
Anteroposterior (AP) radiograph of a left knee with an implanted dynamic spacer (a). Lateral radiograph of the same knee with the implanted dynamic spacer (b)
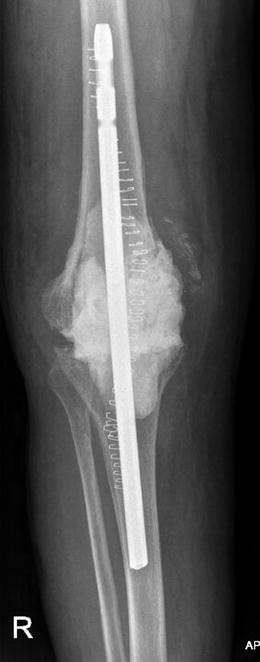
Fig. 18.2
AP radiograph of a right knee with an implanted static spacer
Several antibiotics can be added to the bone cement and should be selected based on the pathogen sensitivity. Synergistic elution activity has been seen with vancomycin and tobramycin [50]. These antibiotics also have thermal stability and come in powder form, which are important factors for successful spacer construction. Contrary to antibiotic cement used for prosthesis fixation, antibiotic-loaded cement spacers combine 2–4 g of vancomycin and 2.4–4.8 g of gentamicin per 40 g bag of cement [51]. Adding more antibiotics to the cement decreases the strength of the cement, but since this is a temporary spacer, increased antibiotic elution is desirable [52–54]. Different antibiotics at different concentrations have been proposed, as stated in Table 18.1.
Table 18.1
Antibiotics and their concentrations used with success results in knee spacers
Antibiotic | Dosage of antibiotic per 40 g of cement | Reported success rate | Reference |
|---|---|---|---|
Tobramycin/vancomycin | 3.6 g/2.0 g | 91–92.4 % | [55] |
Gentamicin/clindamycin/vancomycin | 1 g/1 g/2 g | 95.8 % | [56] |
Tobramycin/vancomycin | 1.2–2.4 g/0.5–1 g | 92.1–94.7 % | [47] |
Tobramycin/vancomycin | 1 g/1.5 g | 86.7–93.8 % | [57] |
Vancomycin/tobramycin | 4 g/4.6 g | 93.5 | [58] |
Vancomycin/gentamicin | 2–4 g/2.4–4.8 g | 92 % | [51] |
Prior to reimplantation, a course of 6 weeks of antibiotics is often prescribed, followed by an antibiotic-free holiday to verify if the infection has been controlled. The total duration between the two stages is a matter of ongoing controversy [59]. There is no consensus for the optimal time period between stages, as reports vary between days and up to several months [8], and Insall (1983) described a 6-week interval as appropriate [36]. There are no definitive markers for reimplantation, but serological erythrocyte sedimentation rate (ESR), serological C-reactive protein (CRP), and synovial fluid leukocyte count [60, 61] should decline or reach normal values prior to reimplantation. Synovial fluid cultures can also be of value, although they can be falsely negative and misleading [62]. Lastly, other markers such as leukocyte esterase and alpha-defensin may also aid with the timing of the second surgical stage [63, 64]. The second stage consists of a final reimplantation and prosthetic reconstruction.
18.3.4 Suppression Antibiotic Therapy
Suppression antibiotic therapy is usually reserved for patients who are not suitable for or refuse to undergo surgical treatment. The literature is limited on this topic, but some authors have shown that suppression antibiotic therapy could be a viable option in candidates, when surgical risks outweigh the benefits. These patients must have a stable prosthesis, must have absence of systemic infection, must have an identifiable pathogen sensitive to oral antibiotics, and must be able to tolerate oral therapy. Suppressive antibiotic therapy can also be used in patients that have persistent, latent infections due to various reasons, including component retention. Studies that evaluate the duration of treatment fail to propose an optimal time frame because therapy alters inflammatory markers and side effects might affect compliance.
This treatment option is considered suitable, with favorable results in selected patients [65, 66]. Overall success rates have been reported as low as 23 % and up to 86.2 % [12, 67, 68]. Reported success varies substantially and no agreement in treatment duration has been achieved. One study that evaluated chronically infected patients that were followed for 3 years observed a success rate of only 23 % [68]. Another study that evaluated a similar patient cohort reported a much higher success rate of 86.2 % [67]. This second study reported that patients with failed treatment were more likely to be infected by Staphylococcus aureus (4 out of the 5 failures), although 69 % of Staphylococcus aureus patients treated with chronic antibiotic suppression were successful [67]. It seems that heterogeneity in the patient populations may be responsible for the variability of outcomes.
Furthermore, suppression antibiotic therapy has been associated with adverse effects. Diarrhea, pseudomembranous colitis, delayed hypersensitivity reaction, nephrotoxicity, leucopenia, and skin discoloration are the most common side effects [12, 67]. Further research is required, however, for the optimal timing, but antibiotic suppression is a viable option for patients for whom surgery may not be a viable option.
18.4 Conclusion
Treatment of PJI in TKA patients remains controversial and poses a great challenge for everyone involved. There are different options for treating PJI after TKA, and surgeons should have full knowledge of treatment options, limitations, and reasons for failure. Host factors, the microorganism present, and surgeon experience are key factors for determining the optimal treatment modality. Involving the patient in the treatment process by explaining the available options is a key, as patient compliance dictates treatment success. PJI treatment must be carefully planned and executed; however, prevention is the first and most important step to successfully eradicate PJI.
References
1.
Diaz-Ledezma C, Higuera CA, Parvizi J (2013) Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res 471:2374–2382PubMedCentralPubMedCrossRef
2.
Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J (2010) Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 468:52–56PubMedCentralPubMedCrossRef
3.
4.
5.
6.
Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ (2012) Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res 470:130–137PubMedCentralPubMedCrossRef
7.
8.
9.
10.
11.
12.
Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR (2006) Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 2003:471–478CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








