(1)
Department of Orthopedic Surgery ASAN Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of South Korea
Abstract
Arthroplasty is probably the most variable procedure as the techniques vary according to the patients’ morphology and the severity of arthritis.
Good results cannot be achieved without careful planning even in primary arthroplasty, especially when there are accompanying problems such as bone defects, deformity, and/or instability of the knee joint. Although such conditions are more frequently encountered during revision arthroplasty, I feel the need to share a separate chapter for describing the specific extraordinary surgical procedures so as to avoid their omittance.
This chapter comprises of the specific conditions and/or diseases of the knee joint that need special attention.
5.1 Bone Defects
5.1.1 Introduction
Bone defects can be found in various disorders or conditions of the knee joint. In primary TKA, bone defects are commonly observed in the medial or posteromedial side of the tibia if there is a varus deformity due to severe degenerative arthritis or in the lateral condyle of the femur if there is a valgus deformity due to rheumatoid arthritis (RA) or traumatic arthritis, etc. Bone defects can also develop in case of osteonecrosis, after high tibial osteotomy or Charcot disease. During revision arthroplasty, bone defects are encountered in cases with infection and loosening, and they may also develop while removing the prosthesis and bone cement.
5.1.2 Classification
Bone defects are classified according to their site, size, and shape. They can be classified using a simple and easy method into contained (cavitary) defect and uncontained (peripheral, segmental) defect; a contained defect is a cavitary defect surrounded by an intact cortical or corticocancellous bone, and an uncontained or peripheral defect is a defect in which the cortical rim is deficient (Fig. 5.1).
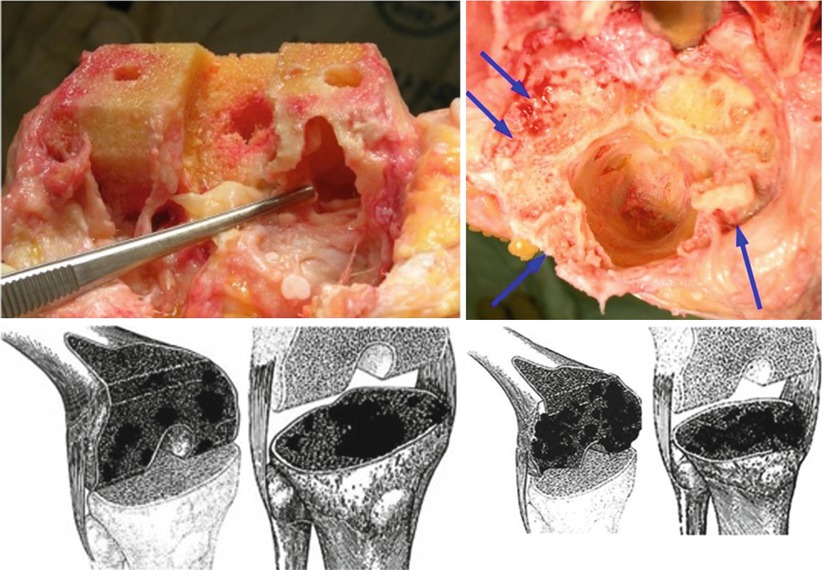

Fig. 5.1
Contained and uncontained (peripheral) defect
However, the AORI (Anderson Orthopaedic Research Institute) classification, which classifies bone defects according to the size and shape in addition to the contained and uncontained defect, is the most widely used classification. The AORI classification is systematic, and it can be applied to both the tibia and femur. This classification can be made preoperatively based on the X-rays, but it is more accurate to classify the bone defects intra- or postoperatively since the classification may change during operation.
This classification is based on the condition of the bone defect in the metaphyseal segment. According to this classification, bone defects are divided into three types: type I, II, and III. Femoral defect is divided into F1, F2, and F3, while tibial defect is divided into T1, T2, and T3 (Fig. 5.2).
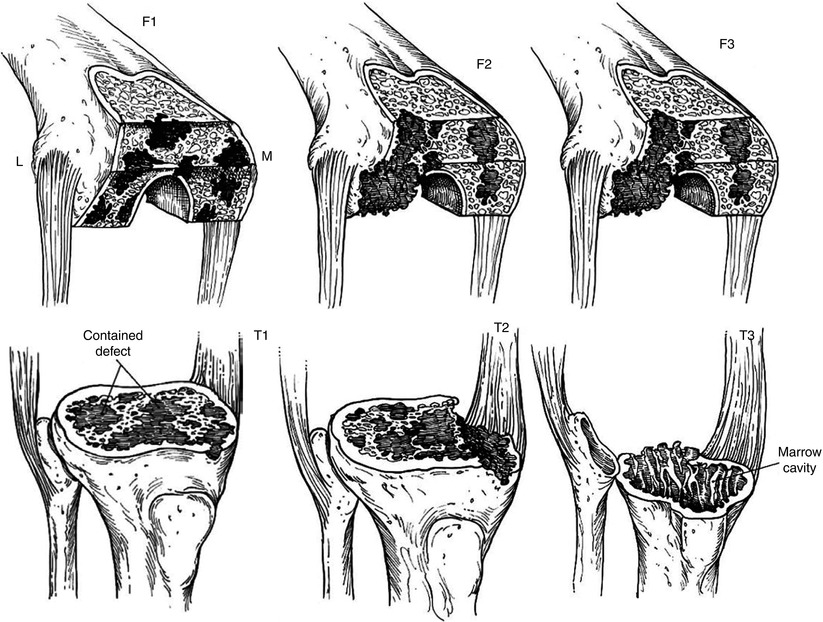

Fig. 5.2
The AORI bone defect classification
Type 1 defect, i.e., F1 or T1, has an intact metaphyseal bone and refers to minimal bone defects that do not affect the stability of prosthesis. Type 2 defect, i.e., F2 or T2, has an epiphyseal defect of the cortical or cancellous bone. If this type of bone defect is found only in one of the tibial or femoral condyle, it is classified as type 2A, whereas type 2B refers to bone defects in both condyles. Type 3 defect, i.e., F3 or T3, has a deficient metaphyseal segment, and generally the collateral ligaments or the patellar tendon has been detached from the bone or has been injured since the main parts of the femoral condyle or the tibial plateau are missing.
The major differentiation between the type 2 and type 3 defects is whether the joint stability can be maintained by the usual bone reconstruction methods alone or not. Anatomically, the femoral defect distal to the epicondyle is type 2, and the femoral defect proximal to the epicondyle is type 3. The tibial defect proximal to the fibular head is type 2 and that distal to the fibular head is type 3. In type 1 defect, patients have little deformity or instability, whereas the patients with a type 2 defect have a varus or valgus deformity, and those with a type 3 defect always have instability due to insufficient ligamentous structures.
Bone defects may also occur in the patella, but they are excluded from the AORI classification.
However, the AORI classification is sometimes not practical as it does not consider huge cavitary defects which are frequently encountered during revision surgery. Hospital for Special Surgery (HSS) introduced a new classification, which divides bone defects into cystic, epiphyseal, cavitary, and segmental defects in the cancellous bone. This method of classification is more practical for determining the bone reconstruction method during revision surgery. The cystic defect is a small contained defect and does not affect the stability of prosthesis; bone cement is used if the diameter of the defect is 5 mm or smaller, and bone graft is performed if the diameter of the defect is greater than 5 mm. The epiphyseal defect is a bone defect involving the cortical bone of the epiphysis or metaphysis and may affect the stability of prosthesis. Usually there is a wedge-shaped defect on the medial tibial plateau, and it is observed when there is a varus deformity. This type of bone defect can be reconstructed using metal augments, and an extension stem may be needed in some cases. Cavitary defect involves a severe loss of the cancellous bone although the cortical wall remains intact, and it is caused due to severe osteolysis, infection, or during removal of the prosthesis with a violent force. It affects the stability of prosthesis, and hence, strut allografts or impaction of morcellized grafts with extension stem or trabecular system may be required. Segmental defect is a combined epiphyseal and cavitary defect, and it may be required to use allografts or hinged prosthesis.
5.1.3 Diagnosis
In primary TKA, the diagnosis of bone defect can be made relatively easily on the simple preoperative X-rays, and the classification of bone defect rarely changes when the operation has been performed properly. The AORI type I defect may not be detected on the simple X-ray. In the type II defect, there is an accompanying varus or valgus deformity of the knee joint on weight-bearing A–P X-rays. The knee joint may also show slight subluxation. In primary osteoarthritis, the bone defect is generally on the posteromedial side of the tibia. On careful observation, two joint lines can be seen on the A–P view, and these lines are seen more clearly on the lateral X-rays (Fig. 5.3). In femoral defects, the femoral condyle shows some flattening on the A–P and the lateral view, and this finding becomes more obvious when lines are drawn along both the condyles on the lateral view. In type III defect, the bony defect is clearly seen, and the deformity is marked on the simple X-ray. The instability is more prominent on weight bearing or stress X-ray.
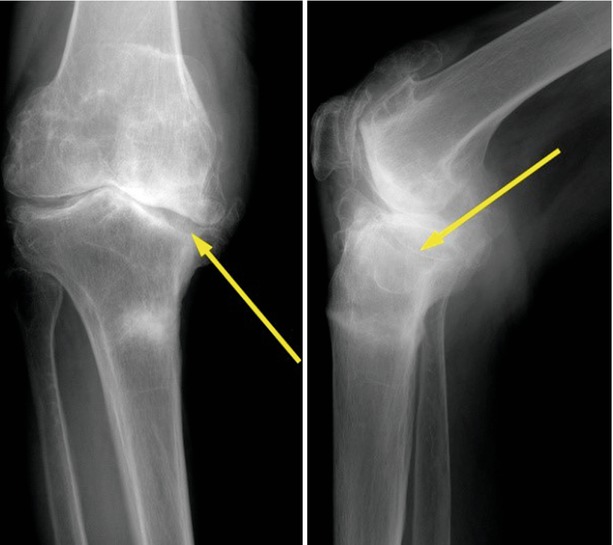

Fig. 5.3
X-ray of the bone defect in the tibia (arrow)
In revision TKA, it is not easy to make the diagnosis of bone defect on simple X-ray. In osteolysis or loosening, bone defect is often masked because of the overlap of bony contour. CT or three-dimensional (3D) CT is often needed in revision cases. Also, the bone defect classification can change during or after the operation since implant removal may have an effect on the extent of bone defect.
5.1.4 Principles and Methods of Treatment
The purpose of bone defect reconstruction is to achieve implant stability with good alignment, maintain the joint line, and achieve an adequate ligament balance. The principles of treatment depend on the type and size of bone defect, but other factors such as patient’s age, activity, or life expectancy are also considered.
When bone defects are divided into contained and peripheral defects, the contained defect is reconstructed using either morcellized cancellous autogenous or allogeneic bone grafts or by filling the defect with bone cement if it is a small defect. Whereas structural allografts or trabecular system may be needed for larger contained defects. Scott emphasized that a bone defect caused by a large cystic cavity in rheumatoid arthritis or osteoarthritis (OA) should be filled with bone graft after curettage. He stated that when a cystic defect is filled with bone cement, there is a risk of developing loosening due to progressive bone resorption.
In a peripheral defect, several methods such as metal augmentation, bone graft, custom-made prosthesis, tumor prosthesis, or APC (allograft prosthesis composite) can be used according to the size of bone defect.
Principles of treatment according to the AORI classification are as follows: In a type 1 defect, it is only required to fill the defect with a bone graft or bone cement. In a type 2 defect, it is required to use bone cement, metal augment, or bone graft, and extension stem may be required to prevent subsidence or migration of the prosthesis. Type 3 defect can be reconstructed with bone graft alone (Fig. 5.4 ), bone graft along with metal augmentation or trabecular system, and a constrained type or hinged prosthesis may be used if the implant stability seems doubtful. In severe bone defects, the use of tumor prosthesis or custom-made prosthesis can be considered.
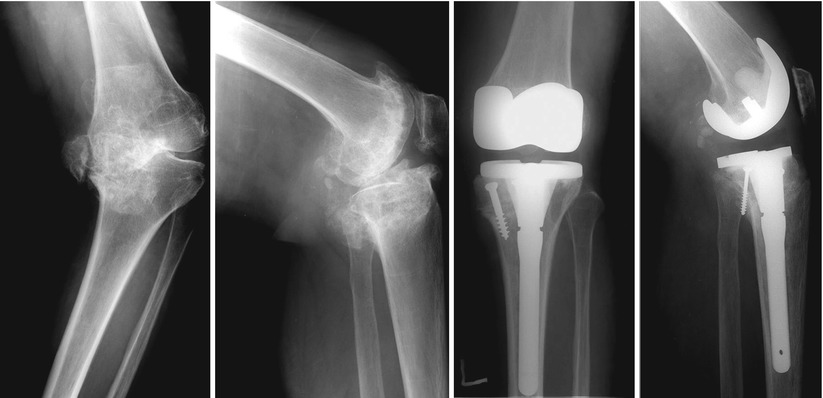

Fig. 5.4
AORI T3 defect reconstructed using structural allograft
I agree with the above principles of treatment, and I would like to describe the methods in more detail.
5.1.4.1 Cementing
Cementing is recommended when the bone defect is smaller than 5 mm in depth regardless of whether it is a contained defect or a peripheral defect. Chen and Krackow demonstrated that cementing is mechanically as strong as metal augmentation in smaller bone defects since the cement fills the defect completely. Cementing is not recommended in relatively younger and active patients. Considering revision surgery, cement is mechanically weaker than bone graft, and bony incorporation occurs easily in younger patients.
When there is a slightly larger peripheral bone defect, cementing and screw augmentation techniques may be used. Ritter and Harty found that cement with screw augmentation showed superior clinical results than those in cement without screw augmentation. Scott introduced screw augmentation in the distal part of the femur and when the appropriate length of screws is used, they can maintain proper alignment as well as strengthen the cement fixation. The technique requires insertion of screws into the bone and covering them with cement. The use of titanium cancellous screws is recommended, and it is necessary to ensure that the screws do not protrude from the cement (Fig. 5.5).
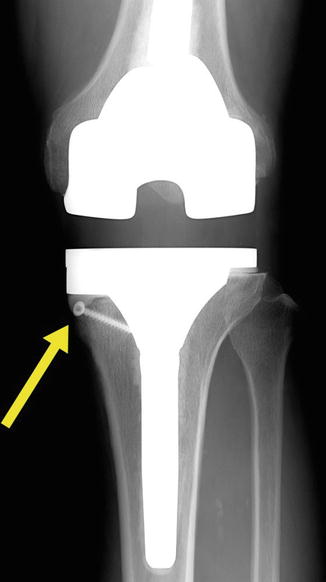

Fig. 5.5
An X-ray of cement with screw augmentation technique
On the other hand, according to Brooks et al., cementing of larger bone defects worsens the clinical results, and radiolucent lines appear beneath the cement. The reason for this is that larger lumps of cement cause thermal necrosis, infection, and fibrous membrane formation. However, Pagnano et al. insisted that osteolysis is the result of poor cementing technique, and they emphasized the importance of proper cementing technique.
It is important not to make a wedge-shaped cut but to make a step cut on the host bone so as to reduce the shear force, especially in a peripheral defect. Chen and Krackow experienced 100 % failure when cement was formed into a wedge shape.
5.1.4.2 Metal Augmentation
Metal augment is convenient and simple to use. Brand et al. observed good results using metal augments without radiolucent lines after 5–6 years of midterm follow-up.
There are two types of metal augments, wedge or flat block (rectangular) type for the tibial defect and single unit that augments the anterior, distal, and posterior defects simultaneously or separate blocks that augment the femoral defect according to their location.
Wedge Type
The wedge type of metal augment is an earlier model, and its major benefit is preservation of more bones, since most of the bone defects are wedge shaped. However, more time is needed to perform osteotomy for an accurate engagement of the augment into the defect. Another drawback of the wedge type of metal augment is that the augment is under shear force instead of compressive force (Fig. 5.6). For this reason, Windsor et al. recommended the use of an extension stem with a wedge-shaped augment.
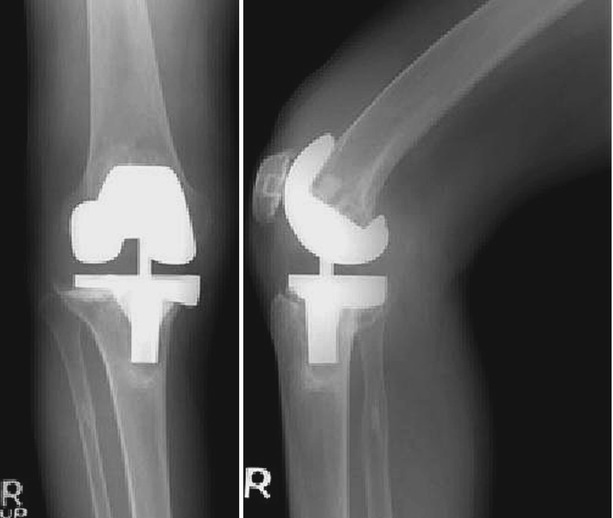

Fig. 5.6
X-rays showing loosening in wedge type of metal augment
Block Type
On the other hand, although a little more bone resection is required for using the block type of metal augment, it is easier to apply, and it is mechanically more durable than the wedge type of augment. This finding was confirmed in studies by Fehring et al. and Cho et al.
The fixation of metal augment onto the implant is done either by using screws or cement. Cement fixation can reduce metal corrosion; however, rigid fixation cannot be achieved, and changes in the position of the augment can occur. Screws can provide rigid fixation between the augment and the implant, but they may cause corrosion due to metal interaction. Hence, it is desirable to use both the fixation methods.
Extension stem is needed to a lesser extent with the block type of metal augment than with the wedge type, but it is desirable to use an extension stem when a thicker augment is used or when the stability of prosthesis is doubtful due to osteoporosis.
Trabecular System
If a cavitary defect is huge, a metal trabecular system may be used to reconstruct the bone defect. It is made of porous tantalum and is designed for bony ingrowth. The advantage of the trabecular system is that the incidence of infection is reduced and bone resorption does not occur. Its disadvantage is that the surgical technique is not easy as morcellized bone graft may be needed sometimes for tight fitting, and its removal is very difficult during revision surgery. There are various sizes and shapes of the trabecular system according to the shape of the bone defect. Choosing the correct size and shape of the trabecular system is also a very important procedure.
5.1.4.3 Bone Graft
An autogenous graft, allograft, or both can be used in combination as a bone graft. Once the bone graft is incorporated, it is the most biologic reconstruction. This is the reason why bone graft is recommended for younger and more active patients, even though the bone defect is mild. Dorr et al. stated that the use of a bone graft is indicated when surface defects are more than 50 % and are deeper than 5 mm in one condyle.
A contained defect can be treated with morcellized cancellous graft, but a peripheral defect requires a structural graft. Peripheral defect can be reconstructed using impaction allograft with wire mesh.
Autograft
Autogenous bone can be obtained from the osteotomized bones; however, only a limited amount of bone is available.
Morcellized cancellous autogenous graft is commonly used to fill a small bone defect or it is used in combination with allograft. Bones from the distal femur can be used in case of a wedge type of bone defect in the tibia, but autogenous bone is often not suitable for structural bone graft because it is too small for achieving stable fixation (Fig. 5.7), and the bone quality is poor due to sclerotic changes. Iliac bone can also be used, but it can cause donor site morbidity, and its mechanical strength is not adequate when the shape and direction of the graft are not compatible with the host trabecular system.
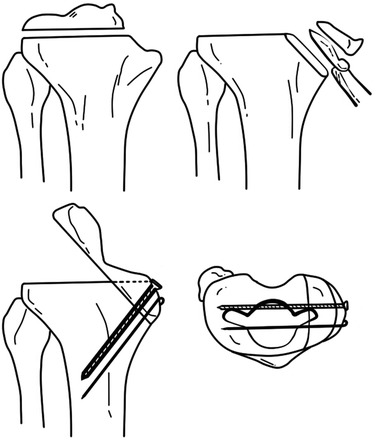

Fig. 5.7
Autograft in the proximal tibia using resected femoral bone
Allograft
Although the allograft has a lower capacity for osteoinduction than the autograft, it has excellent osteoconduction function. Bony incorporation of the allograft can occur, although it takes a longer time than that of the autograft.
Although the allograft has the potential to transmit infectious diseases, it is most commonly used for large bone defects since the required amount of bone can be obtained easily. The femoral head is the most commonly used allograft, while the other bones are also available for this purpose. The femoral head is spherical in shape and can be cut to adapt to the shape and thickness of the proximal tibial and distal femoral defects. The allogeneic bone should be freeze dried in order to reduce immune reactions.
The problem with the use of allograft is that it occasionally collapses when it fails to incorporate into the host bone. Also, the allograft undergoes creeping substitution after revascularization, and this process may cause nonunion if it occurs too slowly, or a fracture may develop if it occurs too rapidly. Even after bone union has been achieved, if the graft bone is too large, it acts as an inert material causing fatigue fracture over time due to limited remodeling.
Bone Graft Techniques
A few factors are important for enhancing the incorporation of the bone graft. First, good alignment of the implant must be ensured. Second, cancellous to cancellous interface contact should be achieved. Third, rigid fixation should be established. Fourth, interposition of cement between the graft and host bone should be avoided. Lastly, the sclerotic bone should be drilled to cause bleeding. However, some surgeons do not recommend drilling of the sclerotic bone since the sclerotic bone plays an important role in providing bony support.
Contact
The complete removal of soft tissues and foreign bodies is as important as avoiding contact of the cortical allograft to the cancellous host bone. The contact surfaces of the graft bone and the host bone should be even and gap-free; hence, an osteotomy should be performed such that the grafted bone fits accurately on the host bone, or morcellized bone should be used to fill the gap between the bone graft and the host bone.
Size and Shape
When the morcellized graft is to be used, the bone should be broken into pieces smaller than 5 mm. The structural graft has to be fixed with K-wires or metal screws, and hence the size of bone must be large enough, at least 1 cm in thickness in every plane. If it is smaller than this, the grafted bone can get fractured during fixation. However, if an allogeneic bone is too big in size, it results in problems of union or incorporation and is likely to collapse. The bone graft itself should provide stability by its own shape, and it is recommended that the host bone should be prepared in a reverse trapezoid shape or step cut so as to prevent shear force (Fig. 5.8).
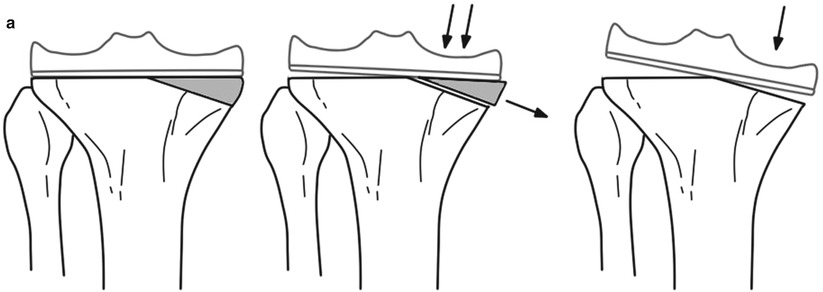
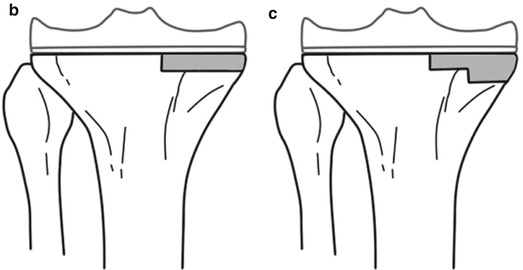


Fig. 5.8
Ideal shape of the bone graft. The bone graft should not be as shown in the top (a) figures, but it should be as shown in the bottom (b, c) figures
When using the femoral head, the acetabular reamer can be used to prepare the recipient site so as to match it with the femoral head.
Author’s Method
In case of a severe segmental bone defect in the tibia, I use the allograft with “the bone in the host bone” method, with which some portion of the patellar tendon and the collateral ligament can be preserved. Allogeneic tibial bone graft can be used in tibial defect just like APC (allograft prosthetic composite), but this allograft does not engage into the host tibia due to the similarity in size of the medullary canal. So there are many difficulties in fixation of the allograft bone to the host bone. As the diameter of femoral diaphysis is smaller than that of tibial metaphysis, inserting the distal part of femur into the tibial metaphysis in the reverse direction is possible (Fig. 5.9). Since the femoral condyle articulates with the tibial plateau, the cut surface of the femoral condyle matches well with that of the tibial plateau.
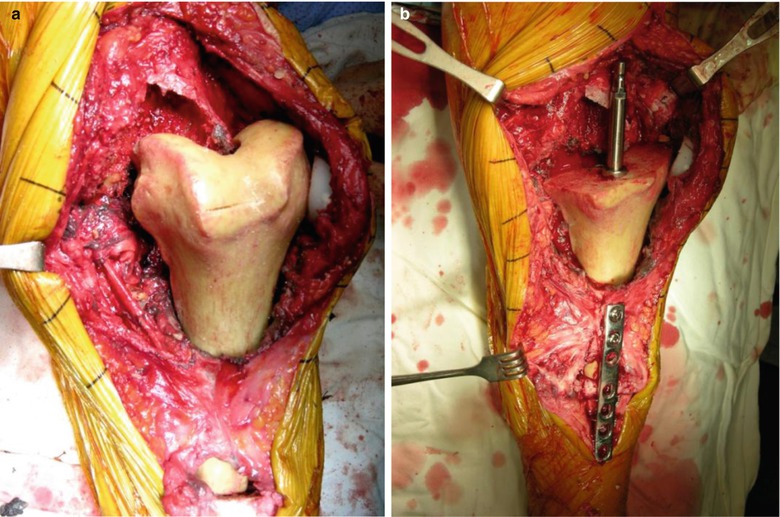
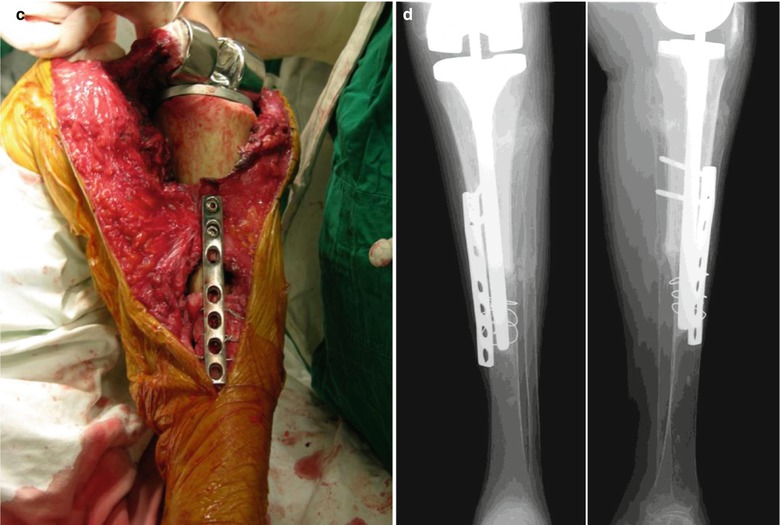


Fig. 5.9
(a) Allograft with the whole distal part of femur inserted into the tibia in the reverse direction for reconstructing a severe segmental defect in the tibia. (b) Plate fixation and reaming. (c) Replacement. (d): Postoperative X-ray
Fixation of Bone Graft
Before morcellized bone grafting, a stem or guide rod is inserted into the intramedullary canal so that the morcellized bone fragments do not enter into the medullary canal. After impaction, carefully remove the stem or the rod, and the prosthesis is inserted (Fig. 5.10). Cement coating on the surface is optional.
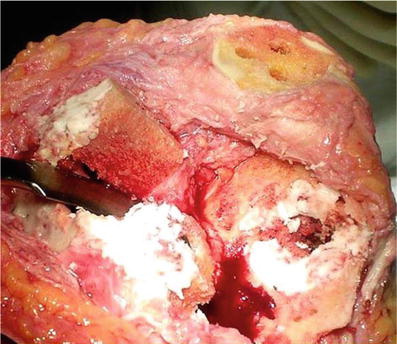

Fig. 5.10
Morcellized bone graft coated with cement
Technically, morcellized bone grafting is relatively easier to perform in the tibia, but it may scatter when used in the femoral side even when it is coated with cement. Hence, the bone graft should be handled with care until the prosthesis is inserted.
A structural graft can be fixed by various methods. K-wires are not recommended for use due to insecure fixation and chances of migration unless they are being used for temporary fixation. Metal screws can be fixed in an oblique or vertical direction. In any case, the screws should not inhibit prosthesis insertion, so it is recommended to place a reamer inside the canal and aim the drill in the direction such that the screws do not interfere with implant insertion. Plate and screws can be used for further fixation.
Author’s Method

While performing morcellized bone graft, I usually release the tourniquet after impaction of the morcellized bone graft in order to allow hematoma formation which prevents the scattering of bone fragments, instead of coating the graft with bone cement.
Revision surgery takes longer time than primary TKA, and twice tourniquet time is usually required. Hence, I release the tourniquet at the time of morcellized bone grafting even though it is not until the time limit of the first tourniquet time.
Sequence of performing morcellized grafting is as follows: fill the defect with a morcellized bone graft, insert the trial implant for impaction, and release the tourniquet so that the blood will flow into morcellized bones to form hematoma. This hematoma holds the morcellized bone fragments together and blocks the penetration of cement into the bone fragments (Fig. 5.11).
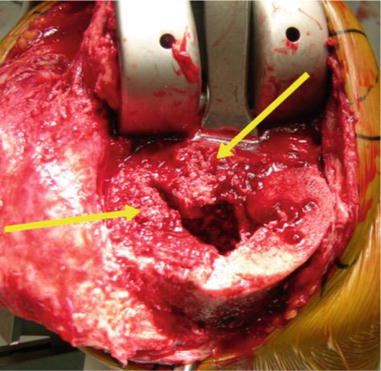

Fig. 5.11
Defect is filled with morcellized bone graft and tourniquet is released. Cancellous bone is held in its place by hematoma
As the hematoma has a limited adherence effect, it is recommended to ask the assistant to press the periphery of graft with his/her hands when the prosthesis is being inserted so that the morcellized bone graft is not squeezed out.
Author’s Opinion
I have a strong doubt about the function and role of allogeneic bone graft.
The advantage of allogeneic bone graft is physiological bone reconstruction. Hence, I performed allogeneic bone grafting in the case in which the bone defect could not be reconstructed using metal augments. I found very disappointing findings during re-revision surgeries of patient in whom allogeneic bone graft was used. The allogeneic bone graft appeared just like an inert bone mass rather than a living bone, and the bone quality was also poor. I had the opinion that allogeneic bone graft was not better than bone cement.
On the other hand, I have experience of filling large bone defects with cement. Clinically, the patient showed no specific complications at the 3-year follow-up. Considering that the use of allogeneic bone graft is associated with the risk of disease transmission, prolonged operation time, incorporation failure and increased costs, the routine use of allogeneic bone in reconstruction of bone defects should be reconsidered.
I have developed a new cementing technique, which is a modification of screw augmentation. After preparing the host bone with a burr, I insert multiple cancellous screws at the defect site. The screw heads are located just beneath the implant. Cement is filled at the site of screw insertion and extension stem is used for additional fixation. This technique is useful in the elderly and less active patients. The advantages of this technique are that it is easy to perform, it is cost-effective, there is no risk of disease transmission, and early weight bearing can be allowed (Fig. 5.12).
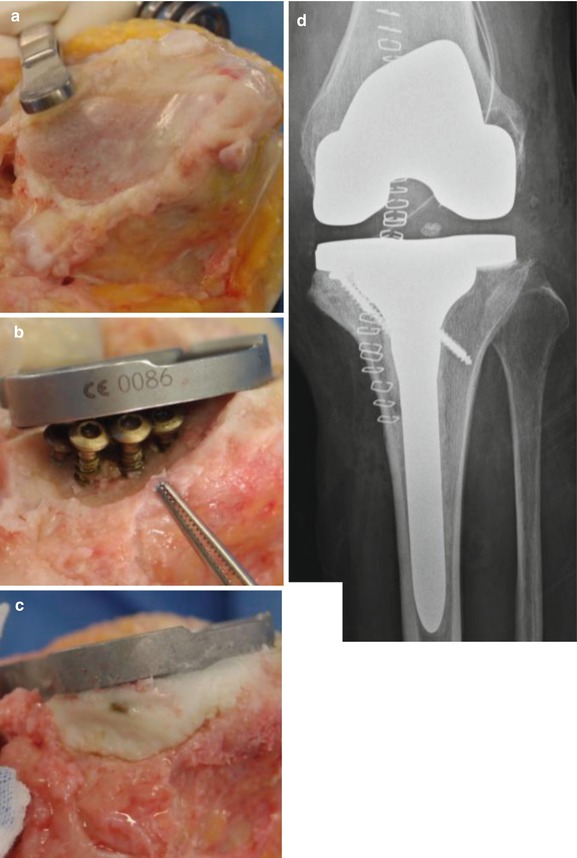

Fig. 5.12
Bone defect reconstruction with cement using multiple screws augmentation. (a) Bone defect. (b) After multiple screw insertion. (c) After cement fixation. (d) Postoperative X-ray
5.1.5 Surgical Methods of Each Bone Defect
5.1.5.1 Tibial Defect
Tibial defect may exist on the lateral side in case of a valgus deformity, revision, or infection. However, the most frequent tibial defect in primary arthroplasty is located on the medial side.
In case of a small contained defect, the surface is drilled and the defect is filled with cement or morcellized cancellous graft, and if the defect is large, strut cancellous grafting is performed.
For a peripheral defect, the size of the defect is measured and a solution according to the size of the defect is determined. If the real defect is less than 5 mm, it can be reconstructed by cementing alone or cementing with screw augmentation. If the defect is confined to the epiphysis (real defect less than 10 mm), it can be reconstructed with a metal augment. If the real defect is larger than 10 mm, an allograft is needed. As the osteotomy level from the articular surface is different between medial and lateral condyle, real defect implies the defect needed to be reconstructed. When extension stem is used, the posterior slope should be 0°, since the angle of most of the extension stems is designed perpendicular to the tibial plate.
In case of large cavitary defect, trabecular metal augment can be used (Fig. 5.13).
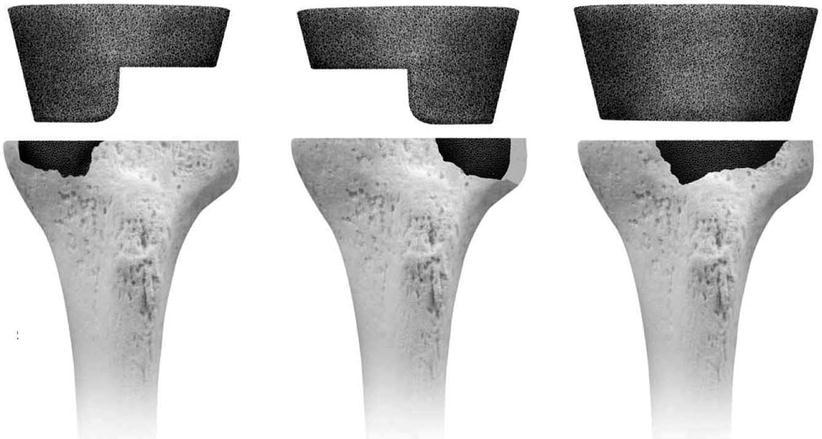

Fig. 5.13
Trabecular metal augment on tibial cavitary defect
Author’s Method
My principles for reconstructing the peripheral defect in the tibial side are as follows:
On the true A–P X-rays, I draw a straight line at right angles to the mechanical axis of the tibia at a virtual resection point (subchondral bone) of the medial condyle. Based on this line, I measure the amount of bone that would be resected from the lateral condyle (Fig. 5.14).
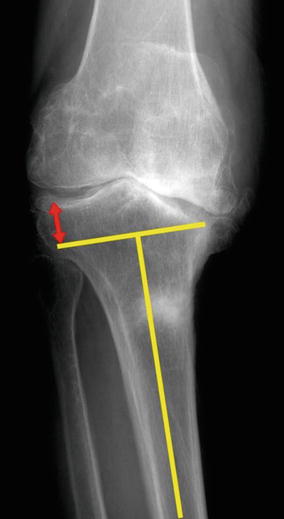

Fig. 5.14
Method for measuring the expected lateral resection in the tibia
I divide the amount of possible bone resection from lateral condyle into four groups: 8 mm or less, 9–12 mm, 13–22 mm, and 23 mm or more, and then I decide how to reconstruct the medial tibial defect. The reason why I use the distance from lateral condyle rather than real defect is that this distance is easier and practical to use. Assuming that about 8 mm of osteotomy level is noted in ordinary TKA, 8 mm subtracted from measured distance is the real defect.
When less than 8 mm of lateral osteotomy is expected, it is considered to be normal. And when bone resection is expected to be another 4 mm, namely, up to 12 mm, either I do an 8–9 mm osteotomy and do cementing of the medial defect or I perform a 12 mm osteotomy of the lateral condyle, and I use a thicker spacer without medial augmentation (Fig. 5.15).

Fig. 5.15
Additional osteotomy and use of thicker PE for T1 defect
Cases in which lateral resection is expected to be up to 13–22 mm, it may be classified into AORI T2. This means that there is a 5–10 mm of real bone defect, and it is reconstructed using a metal augment. The reason why the use of this method is limited to 22 mm is that most of the metal augments can cover up to 10 mm, and a metal augment combined with bone graft is not recommended in case of more than 10 mm of a real bone defect (Fig. 5.16).
If the lateral resection is expected to be more than 22 mm, it can be classified into AORI T3 with a real bone defect of 10 mm or larger than 10 mm. In this case, the use of a structural allograft or tumor prosthesis may be considered.
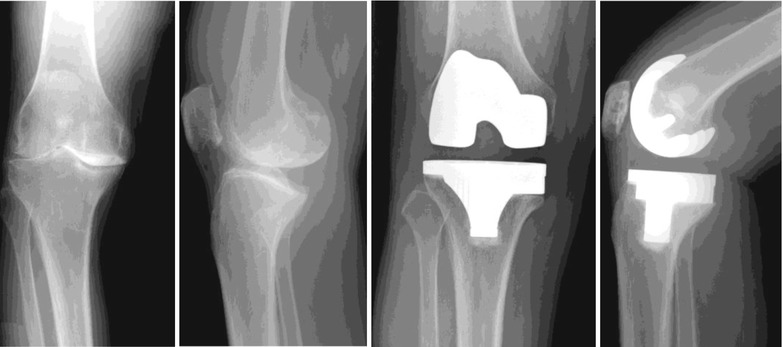

Fig. 5.16
Use of a metal augment in a T2 defect
The reconstruction of tibial defect can be summarized as follows (Fig. 5.17):
(a)
Expected lateral resection of 8 mm or lesser → Routine method without augmentation
(b)
Expected lateral resection of 9–12 mm → Cement augmentation or more bone cutting and using thicker PE
(c)
Expected lateral resection of 13–22 mm → Metal augmentation
(d)
Expected lateral resection of 23 mm or greater → Allograft
(e)
Massive defect → Trabecular augmentation (cavitary)/tumor prosthesis
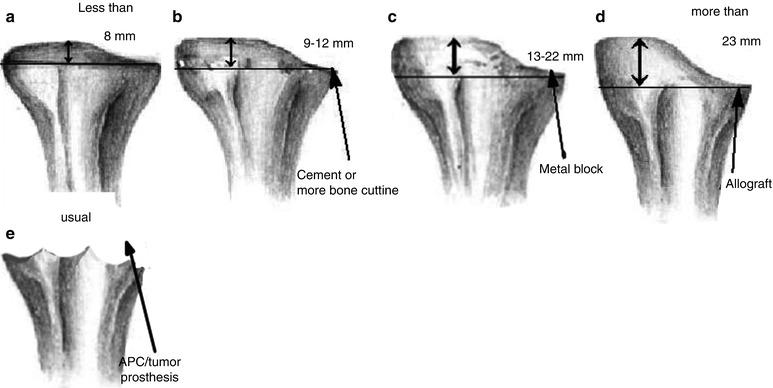

Fig. 5.17
My principles for treatment of the tibial defect
When using structural allograft on the tibial plateau, I prefer to use 4.0 mm cancellous screws to fix the bone graft. The shape of the bone graft is designed so as to facilitate compressive force, and the gap between the allograft and the host bone is filled with morcellized bone. Special techniques are used while inserting the screws, e.g., screw fixation with the stem guide inside the medullary canal and countersinking the screw head to prevent protrusion of screws (Fig. 5.18). If the bone graft is large, oblique insertion of screws or buttress plating can be considered. To prevent a fracture of the grafted bone during implantation of prosthesis, I enlarge the keel site with a saw before using the keel punch (Fig 5.19).
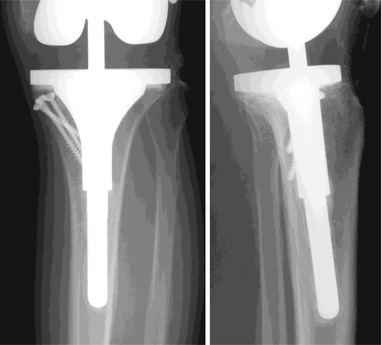
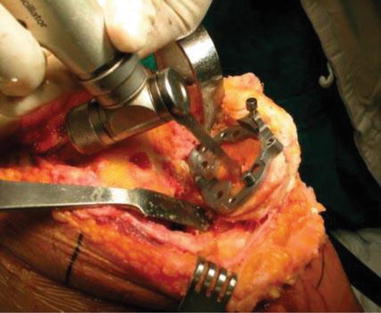

Fig. 5.18
Graft fixation using screws for strut graft

Fig. 5.19
Sawing before keel punching to prevent fracture of grafted bone
Special care should be taken for the correct positioning and rotational alignment of the tibial prosthesis as the grafted bone appears different from the normal bone and may adversely affect implant positioning. For positioning and rotational alignment, the tibial tuberosity should be used as the landmark, or the dynamic method can be used for checking the patellar tracking while flexing and extending the knee joint repeatedly. If this is not done, the prosthesis is likely to be inserted in the internal rotation position, and this may cause patellar maltracking or restrict the flexion motion.
For ensuring stability of the bone graft, the use of an extension stem and cementing is recommended in AORI type 3 bone defect, although opinions of surgeons vary in terms of cementing around the stem.
5.1.5.2 Femoral Defect
Reconstruction of the femoral bone defect is different from that of the tibial bone defect in several aspects.
First, the anatomy of the femur is complex, and it is often difficult to make an accurate diagnosis of the site and size of the femoral defect on simple X-rays. Second, the joint line should be preserved as much as possible. The tibial defect can be reconstructed up to a certain level, and the gap can be adjusted using different PE thicknesses, whereas in case of the femoral defect, a more accurate reconstruction is required in order to maintain the joint line and to balance flexion and extension gaps. Third, rotational alignment should be determined at the time of bone reconstruction. In almost all cases, the anatomical landmarks are lost due to the bone defect and ordinary osteotomy instruments cannot be used. When determining the rotational axis, the use of the posterior condylar axis is difficult and less reliable due to the bone defect. The external rotation should be based on the transepicondylar axis or Whiteside’s line, but very often it may be impossible to use these parameters. Therefore, it is a good option to set external rotation using the ligament tension technique.
The key to bone defect reconstruction is preservation of the joint line and balance the flexion/extension gap, and the amount of bone defect is measured based on the joint line. The landmarks for determining the joint line include the inferior pole of the patella, fibular styloid process, tibial tuberosity, and femoral epicondyles. The X-rays of contralateral knee joint and the pre-marked joint line before removing the implant in revision surgery (this has been described in Chap. 9) can be of great help. The position of the inferior pole of the patella changes during flexion and extension motion which causes the surgeons to make mistakes. The use of fibular styloid process is also difficult as it is located far posteriorly and laterally, while the use of the tibial tuberosity is ambiguous as it is too broad. It is known that the joint line is about 25 mm distal to the lateral epicondyle and 30 mm distal to the medial epicondyle. It is about 20 mm proximal to the tibial tubercle and 14–16 mm distal to the PCL insertion. When the trial implant is inserted at the level of the joint line, the gap between the trial implant and the host bone is the size of the bone defect (Fig. 5.20).
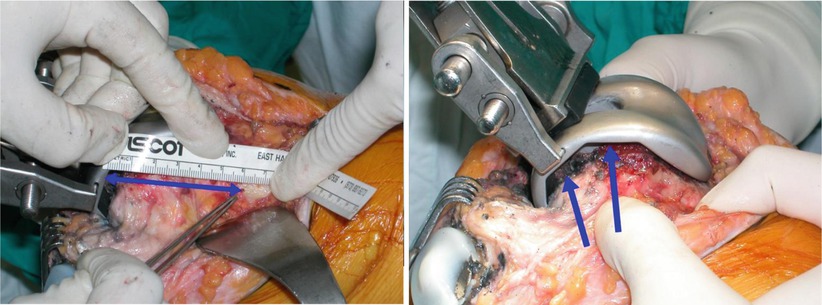

Fig. 5.20
Measuring the joint line before removing the femoral component in revision TKA (left). The trial femoral implant is inserted up to the joint line which is determined by the drill hole made before removal of the implant, and the gap between the implant and the bone (arrows) is the size of the bone defect (right)
The method of reconstructing the femoral defect is not very different from that of the tibial defect. An F1 defect is filled with autogenous slice bone graft or cement. Resection of the distal portion can be done occasionally according to the gap discrepancy. In F2 defect, filling the bone defect with metal augment is a better option than distal bone resection. However, cementing is not recommended as it can cause malalignment. For an F3 defect, an allograft is required, while tumor prosthesis may also be used in case of a massive bone defect.
Author’s Method
Based on these principles, my method of reconstruction of the femoral defect is as follows (Fig. 5.21):
(a)
Bone defect of 2 mm or less → Joint line elevation or slice bone graft
(b)
Bone defect of 4 mm or less → Distal bone graft or more resection and metal augmentation
(c)
Bone defect of 5–10 mm → Metal augmentation
(d)
Bone defect of 11 mm or greater → Allograft
(e)
Massive bone defect → Trabecular augmentation (cavitary) or tumor prosthesis or APC
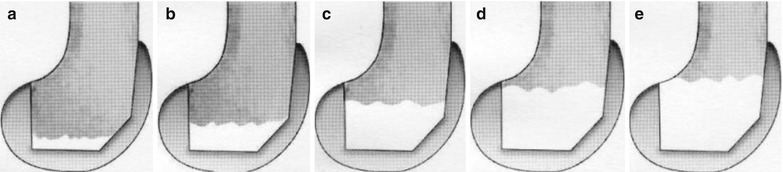

Fig 5.21
My principles for reconstruction of the femoral defect. (a) Less than 2 mm → joint line elevation or bone graft. (b) Less than 4 mm → slice bone graft or more resection and metal augment. (c) 5–10 mm → metal augmentation. (d) More than 11 mm → allograft. (e) Massive defect → tumor prosthesis or APC
When a screw is inserted in the distal to the proximal direction for the fixation of bone graft, countersinking is performed so that the screw head does not interfere with the implantation (Fig. 5.22). Whereas washers provide a more rigid fixation in fixation from the lateral side. I also use the buttress plate when the bone graft is large. In this case, like in the case of tibia, I insert a trial implant before drilling for ascertaining the accurate direction of the screws.
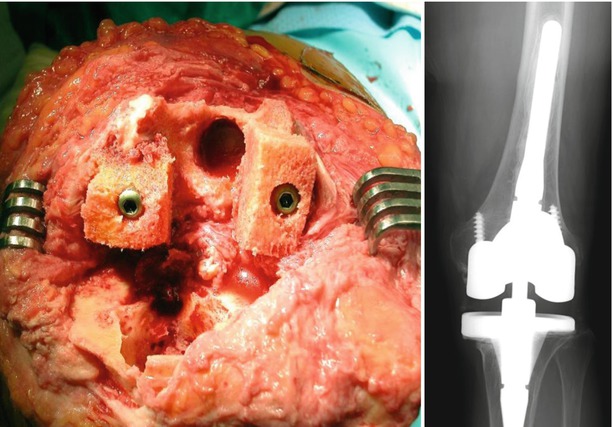

Fig. 5.22
Femoral bone graft fixed with cancellous screws
Anterior femoral defect is not included in the AORI classification although it is found in almost all cases of revision surgery. It occurs due to stress shielding of the anterior part, or its occurrence is inevitable during implant removal. The defect in this portion can be filled with cement, bone graft, or metal augment. Dennis recommended a cementing technique as the bone graft tends to be resorbed due to stress shielding.
Posterior femoral defect can be treated with metal augment, if the defect is large. If the defect is small, it can be filled with cement. In this case, careful attention should be paid to prevent internal rotation of the femoral component.
For stabilization of the implant and bony incorporation, the use of an extension stem can be considered. Extension stem is useful not only for secure fixation, but also for correct alignment. The length of the stem differs according to the method of bone reconstruction and whether cement fixation or cementless fixation is done. When cementless fixation is used, the length of the stem should be at least 100 mm.
When applying the extension stem, a few things need to be considered. First, most of the femoral extension stems have the angle set at 5° or 7° in coronal plane, and hence the distal osteotomy should be done at this angle. Therefore, it is meaningless to calculate the angle between the anatomical and mechanical axis on femoral A–P X-ray preoperatively. Second, the extension stem should not be too thick. In most of the cases, the portal site of the extension stem in the intercondylar area is broad. If medullary reaming is done according to this width, periprosthetic fracture is likely to occur postoperatively due to endosteal thinning in the diaphysis. Reaming of the medullary canal for IM nailing in fracture treatment does not cause serious problems even though there is endosteal thinning as the IM nail extends beyond the site of reaming. However, the extension stem ends at the site of reaming in the diaphyseal area in TKA and may act as stress riser leading to fracture. Lastly, an offset stem may be needed to prevent anterior angulation due to anterior femoral defect and lower position of extension stem which is a common pitfall during revision TKA.
5.1.5.3 Patellar Defect
Patellar defect occurs when there is a bone cyst, in case of severe patellofemoral arthritis due to long-standing patellar maltracking or in case of revision.
The management of patella defect is dictated by the thickness of more than half of the patellar surface after osteotomy. If this thickness is more than 11 mm, then the patellar defect can either be drilled without resurfacing, or the defect can be filled with cement along with resurfacing of patella. If more than half of the patellar surface has a thickness of less than 10 mm after osteotomy, one of the non-resurfacing, biconvex patellae or inset patellae can be chosen (Fig. 5.23). Hanssen stated that pain and function improved after patelloplasty in a huge defect in which bone graft is done, and the graft site is covered with soft tissues. Currently, trabecular metal augmentation has been introduced as a method for reconstruction of a severe bone defect of the patella. Nasser et al. and Ries et al. reported that they could improve function and reduce anterior knee pain by using the trabecular metal augment in patients with severe bone defect (Fig. 5.24). Another method for reconstructing a severe patellar bone defect is the K-wire insertion and cementing technique. Resection patelloplasty by trimming of the remaining bone is performed when there is only a cortical shell of bone remaining. Pavizi et al. observed that this procedure delivers better results than patellectomy or patellar resurfacing. However, Barrack et al. demonstrated that about one-third of the patients experienced anterior knee pain and showed poor satisfaction due to difficulty on stair climbing.

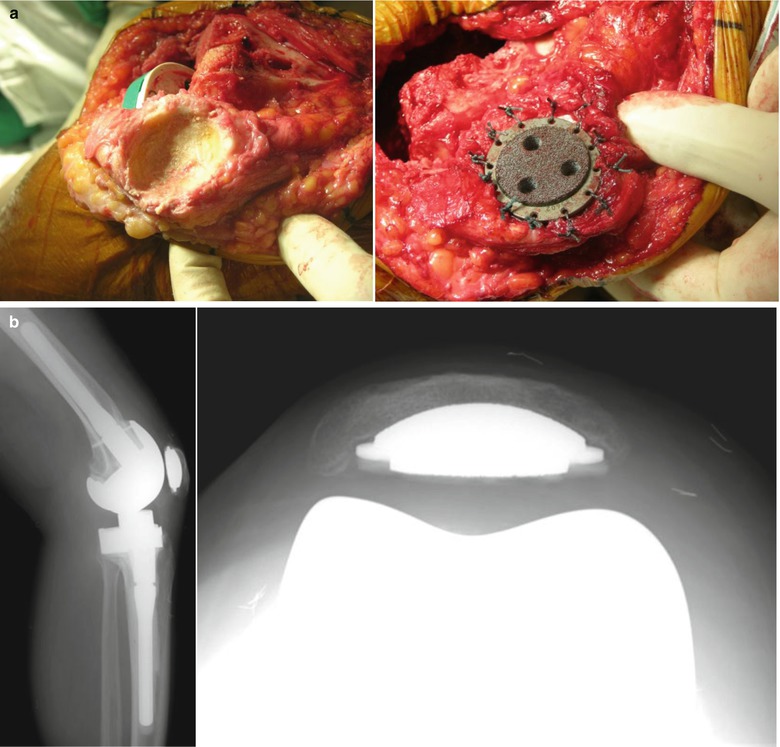

Fig. 5.23
Biconvex patellar component (Smith & Nephew, Richards, TN)

Fig. 5.24
Patellar resurfacing using trabecular metal augment. (a) Operative findings while using trabecular metal augment (b) Postoperative X-rays
Vince et al. introduced gull-wing sagittal osteotomy and bone grafting of the anterior part of the patella in case only a thin cortical shell of bone was remaining (Fig. 5.25).
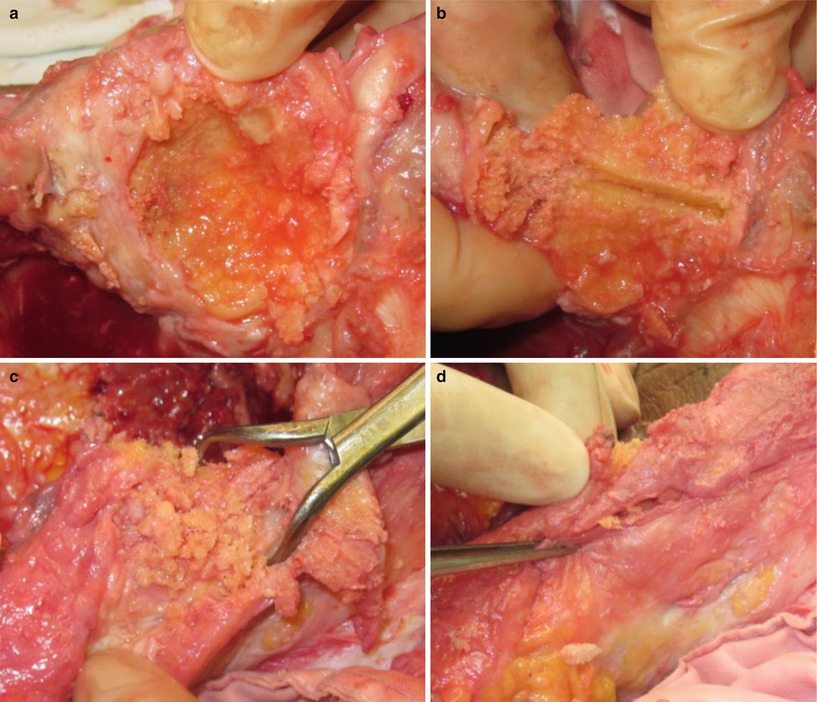

Fig. 5.25
Gull-wing sagittal osteotomy. (a) Cavitary defect of patella, (b) vertical osteotomy is done, (c) morcellized bone graft on the anterior side of patella. (d) The graft is sealed with surrounding soft tissue
Buechel introduced the subsynovial iliac graft by using the synovial envelope for augmenting the bone after resection (Fig. 5.26).
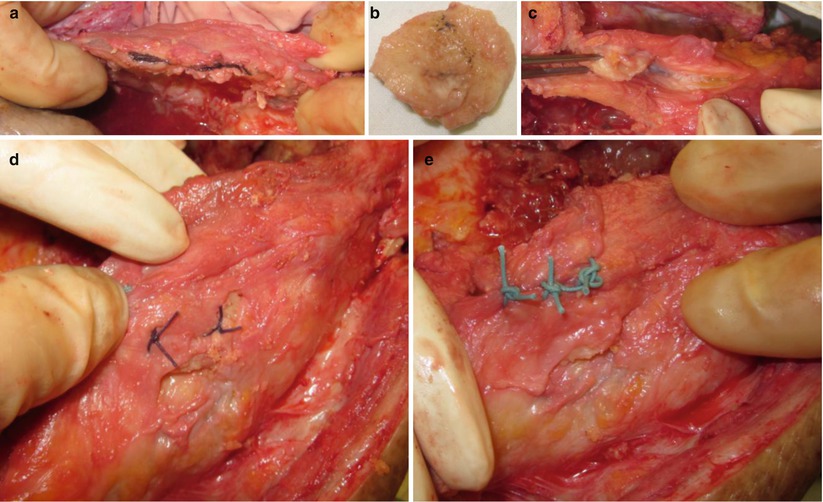

Fig. 5.26
Subsynovial iliac graft. (a) Split the soft tissue on the patella area. (b) Iliac bone is harvested. (c) Insertion of the harvested bone into the gap. (d) The bone is fixed with vertical suture. (e) After completion of the graft
Author’s Method
I replace the patella whenever the remaining bone in more than half of the patellar surface is more than 10 mm in thickness. I drill the sclerotic bone and fill the defect with cement. In case of a larger defect, I either perform patelloplasty and use the trabecular system, or I do not resurface the patella.
I have performed gull-wing osteotomy in a patient with vertical fracture of the patella. The result was promising in this special case.
5.2 Deformity
Almost all knee joints have a deformity to a greater or lesser extent preoperatively, so clearly it is the degree of deformity that matters. Deformity can be classified into intra-articular and extra-articular deformity based on the site of deformity. There are different surgical techniques for correction of the intra-articular and extra-articular deformities. When doing correction of deformity, leg length discrepancy may develop, which results in poor clinical outcome. Lang et al. reported that about 83 % of knees measured showed an increase in limb length after TKA. Preoperative varus alignment was associated with an average lengthening of 5.2 and 8.4 mm lengthening in valgus deformity.
5.2.1 Intra-articular Deformity
This type of deformity is largely caused due to a bone defect or laxity/contracture of the ligamentous tissues. However, in most cases these two types of deformities occur in combination.
The surgical techniques for the correction of deformity have already been described in Sect. 5.1 and in the “Ligament Balancing” section, but I think that it is necessary to briefly review them here.
5.2.1.1 Varus Deformity
In the coronal plane, 4°–7° valgus of the femorotibial angle is considered normal, and a valgus angle of 3° or lesser is considered as a varus deformity, which is coincident with the mechanical axis of less than 0°.
The principle of treatment varies according to the degree and cause of the deformity. If the deformity is mild, the method of treatment is similar to the usual osteotomy and medial release. In moderate deformity, the semimembranosus or pes anserinus tendon is released carefully. Osteotomy is performed according to the usual method of aligning the mechanical axis. If the varus deformity is severe, it should first be ascertained whether the deformity is a dynamic deformity or static deformity. Dynamic deformity is purely due to a lax LCL and it is obvious while walking. A lateral thrust or lateral instability is a common finding when the varus deformity is caused due to lax lateral structures. This condition can be detected on the preoperative weight bearing or stress X-rays (Fig. 5.27). This type of deformity can be corrected by a medial release and by using a thicker PE. On the other hand, static deformity is accompanied by a bone defect and a contracture of medial soft tissues. This type of varus deformity is mostly caused due to a bone defect of the medial tibial condyle, which leads to secondary contracture of the medial soft tissues. Therefore, ligament balancing should be done simultaneously with the reconstruction of bone defect.
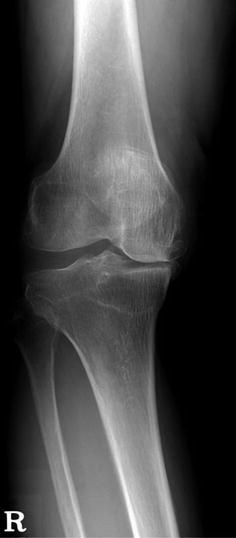

Fig. 5.27
Varus deformity due to lateral instability. Deformity is the result of instability rather than bone defect
There are a few technical tips for the correction of varus deformity. First, the cartilage of the medial femoral condyle may be worn out. So, it is necessary to verify the alignment from time to time to prevent varus alignment during femoral osteotomy. It is also necessary to increase the amount of external rotation when the varus deformity is severe. Second, balance in extension is critical for the varus deformity, while balance in flexion is critical for the valgus deformity. In other words, imbalance in extension causes more problems as a result of instability due to varus deformity, whereas valgus deformity causes more problems in flexion due to contracture of soft tissues. Third, in patients who have had a severe varus deformity since their young age constitutional varus deformity due to such as Blount disease, less correction of deformity is preferable. Otherwise, the patients may fail to adapt to the corrected neutral alignment and suffer from inconvenience or discomfort. Magnussen et al. analyzed the patients with preoperative varus deformities and reported that residual postoperative varus deformity after TKA is not associated with lower International Knee Society (IKS) scores and increased failure rate, provided the tibial component varus is avoided. Fourth, joint instability is likely to occur when the varus deformity is not fully corrected. If ligament balancing cannot be achieved by any of the methods, a constrained implant should be used.
Lateral tibiofemoral subluxation and rotational deformity may develop after correction of a severe varus deformity. Over-release of the medial soft tissues evokes lateral tightening when PE thickness is adapted to the widened medial gap. In such a case, the insertion of the popliteal tendon can be partially released from its femoral attachment to avoid postoperative painful snapping on the lateral aspect of the knee joint.
Author’s Method
I always sacrifice the posterior cruciate ligament (PCL) and release the medial soft tissues until balance is achieved in a severe varus deformity. In many cases, I experienced that the ligament balancing cannot be achieved without complete release of the medial collateral ligament (MCL). Despite of complete detachment of MCL, the implant itself provides stability, and the soft tissues become bound up due to scarring in a balanced state, which eliminates the instability. After balancing has been achieved, the joint motion improves, and stress on the medial part of the knee joint is reduced. If mild instability develops postoperatively, I prescribe brace fitting for 6 weeks until healing of the detached MCL is achieved.
What is most important in complete medial release is that alignment should not be in valgus and the gap in the contralateral side (the lateral side) should neither be tight nor loose. A tight lateral gap further increases the widening on the medial side due to seesaw effect, and a loose lateral gap causes instability on both sides.
I have no experience of procedures such as lateral reefing or advancement, and I believe that it is neither absolutely necessary to perform these procedures nor are these procedures successful. If overcorrection causes an imbalance, I use the constrained type of prosthesis.
5.2.1.2 Valgus Deformity
A valgus deformity is generally caused due to rheumatoid arthritis or post-traumatic arthritis. Ranawat et al. demonstrated that about 10 % of the patients of total knee joint arthroplasty fall into this category. A valgus deformity is sometimes found in both knee joints, but there also is a condition called “windblown knees” in which one knee has a valgus deformity and the other knee has a varus deformity (Fig. 5.28). The mechanism for windblown knees is that the valgus knee pushes the other knee in a high adduction moment and causes a varus deformity of the other knee joint.
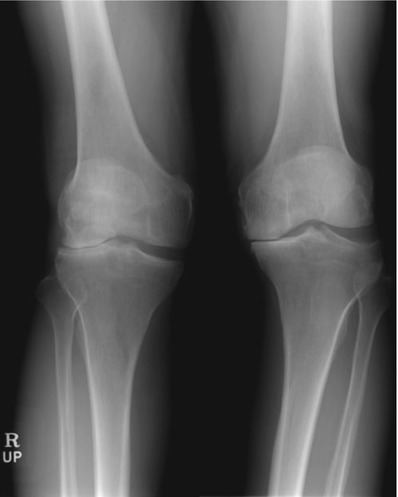

Fig. 5.28
Windblown knees
The valgus deformity has more functional disturbance due to more difficulties in walking and provokes more cosmetic problems. Valgus deformity is not found frequently, and hence beginners would face a completely different situation from that of a varus deformity from ligament release to osteotomy.
The common findings of a valgus deformity are as follows: first, the MCL is lax and lateral soft tissue structures are contracted; second, there often is an accompanying lateral bone atrophy or defect usually in the femur; third, it is also accompanied by the patellofemoral problems. Patellar dysplasia, patellar subluxation, and patella alta may also coexist. The knee joint tends to become unstable due to the MCL relaxation and hyperextension.
Therefore, in a valgus deformity, it is required to release the contracted lateral soft tissues, align the knee through bone resection or reconstruction, and correct the patellofemoral malalignment. The degree of deformity is commonly classified as mild if the valgus is less than 10°, moderate if the valgus is less than 20°, and severe if the valgus is greater than 20°, and step by step soft tissue release is recommended according to the degree of deformity.
Insall suggested releasing the iliotibial band at Gerdy’s tubercle and releasing the soft tissues from the lateral condylar area of the femur in a mild deformity, and followed by resecting the iliotibial band and intramuscular septum in a moderate to severe deformity.
Buechel recommends paramedial arthrotomy when there is a valgus deformity less than 8° and lateral arthrotomy when the valgus deformity is greater than 8°. He recommends performing subperiosteal elevation of the ITB from Gerdy’s tubercle in cases with a mild valgus deformity less than 8° as a first step and proceeding to the second step if valgus deformity becomes 0° with a varus stress and returns to the valgus state between 5 and 8° without stress. The second step is subperiosteal elevation of the lateral collateral ligament (LCL) and popliteus tendon, while the proximal portion is attached to the femur. If the valgus deformity cannot be corrected after the second step, he recommends proceeding to the third step to perform subperiosteal elevation of the LCL and biceps femoris complex at the fibular head or resection of the proximal portion of the fibular head.
Krackow classified the valgus deformity into three types. Type I involves lateral femoral bone loss, lateral soft tissue contractures, and intact medial soft tissues. Type II is type I with lengthened medial soft tissues. Type III is severe valgus deformity with malpositioning of the proximal tibial joint line. He suggested advancement of MCL in type II deformity.
It is controversial as to which ligament should be released first and how much release should be performed. Since the iliotibial band (ITB) is the main structure that can cause the deformity, some surgeons emphasize that it should be released first and the popliteus tendon and the LCL should be retained until the very end, if possible. By releasing ITB, primary correction of the deformity is accomplished by eliminating the main cause of deformity, and the possibility of injury to the peroneal nerve due to excessive retraction is reduced. Releasing can be done by performing pie crust technique, a transverse cut, Z-plasty, or periosteal elevation, and the level of release can be about 8–10 cm above the joint line, at the level of the joint line, or at the Gerdy’s tubercle.
On the other hand, others emphasize that the LCL and the popliteus tendon should be released first from the femur based on the theory that releasing the shorter and closer ligaments affects the gap discrepancy between the medial and lateral sides to a lesser extent in flexion, whereas the longer ligaments open up the gap wider in flexion.
In a severe deformity, it is necessary to release the posterolateral capsule as well as the iliotibial band, along with the LCL and the popliteus tendon. If the LCL and popliteus tendon are not released sufficiently, ligament balance cannot be achieved, and this is similar to medial release in which complete release of the MCL is required until ligament balance is established. The LCL should be completely released by cutting the ligament or through periosteal elevation. Other methods include the lateral cruciform retinacular release. It is also possible to perform sliding osteotomy of the lateral femoral condyle or perform fibular head resection.
Some surgeons suggested minimal soft tissue management and using the constrained type, since there are so many complications due to extensive soft tissue release. Easley et al. reported that primary constrained condylar knee arthroplasty in the elderly patients with valgus knee resulted in significant pain relief and improved function.
It is also controversial whether peroneal nerve exploration is needed during soft tissue release. In fact, the reason to release the LCL on the femoral side is to reduce the incidence of peroneal nerve injury as much as possible, hence it is more acceptable that exploration is not necessary unless there is a severe deformity with a flexion contracture.
The osteotomy is performed as usual following the ordinary classic method of aligning with the mechanical axis. And then the valgus deformity is automatically corrected after ligament balancing. However, distal osteotomy of femur should not be done excessively from the beginning as there is atrophy of the lateral femoral condyle. It needs to be done carefully in order to maintain the balanced joint gap. Conservative femoral and tibial resections are performed, when the deformity is combined with recurvatum or medial soft tissue stretching. Thus, soft tissue balancing can be achieved without elevating the joint line or creating a too large extension gap. Scott emphasized that less than 5° of valgus osteotomy should be done to reduce the tension of the medial side even though the difference between the anatomical and mechanical axes exceeds 5°.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








