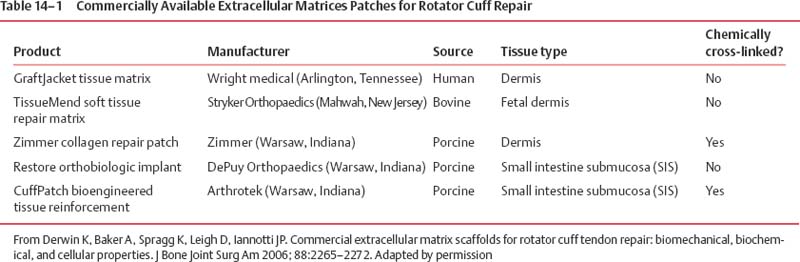
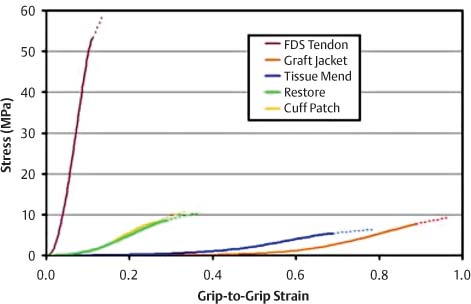
14 Tissue Engineering for the Rotator Cuff–Deficient Shoulder Surgical repair of rotator cuff (RC) tears often results in good to excellent results. However, when evaluated by ultrasound or magnetic resonance imaging (MRI), up to 50% of these tears has been shown to fail to heal.1–6 Many patients improve clinically; nevertheless, results are clearly better in cases where the repaired tendon heals.1,2 Initially, efforts to enhance healing focused on improving mechanical factors, such as the type of suture used, type of knots used, and anchor configuration. Recently, studies have focused on improving the biologic process of healing.7–10 Extracellular matrix scaffolds, growth factors, and gene therapy all may play a role in improving RC tendon healing in the future. In addition, the ideal healing of massive RC tears will involve reversal of the fatty muscle degeneration that accompanies these tears. At this time, extracellular matrices (ECMs) are the most commonly used biologic augments to tendon healing. ECMs are commercially available patches that are Food & Drug Administration (FDA) approved for clinical use for reinforcement of soft tissues that are repaired with suture or suture anchors during RC surgery.3 The scaffolds provide a three-dimensional matrix, which can attract host cells and can provide a site-specific matrix for cell migration. They are resorbable materials around which the body rebuilds more structurally and functionally appropriate treatment. Dejardin’s study,4 in which porcine small intestine submucosa (SIS; DePuy Biologics, Raynham, Massachusetts) was used to treat RC tears in dogs, showed that eventually the patch is reabsorbed from the implantation site; and it is replaced with site-appropriate, host-derived tissue. Because these ECMs are not approved as interposition material to replace absent tendon or to provide the full mechanical strength for the tendon repair, they tend to offer more of a biologic than mechanical advantage with regards to tendon healing. The two main groups of ECMs are those from dermis and those from small intestine submucosa (Table 14–1). Collagen-rich ECMs from small intestine submucosa (SIS) include the Restore Patch and the CuffPatch.5 The Restore Patch (Depuy Orthopaedics, Warsaw Indiana) was the first ECM to receive FDA approval for use in RC repair. It is comprised of 10 layers of porcine small intestine submucosa that has been devitalized so that it theoretically does not contain any viable cells. That being said, a recent study actually confirmed the presence of porcine DNA in Restore.6 It is likely that this is a remnant of tissue processing. The extracellular matrix of the SIS is comprised mainly of type I collagen, fibronectin, chondroitin sulfate, heparin sulfate, and a variety of growth factors, including transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF-2).9–15 Restore is not artificially cross-linked, and it is packaged in a dehydrated form. This contrasts with the CuffPatch (Arthrotek, Warsaw Indiana), which is an eight-layer, acellular, porcine SIS scaffold. Unlike the Restore, following lamination of the layers, the ECM is cross-linked, and it is packaged in its hydrated form. GraftJacket, TissueMend, Zimmer Collagen Repair Patch, and Permacol are ECMs derived from dermis.7 GraftJacket Regenerative Tissue Matrix (Wright Medical, Arlington, Texas) comes from processed human allograft skin from which the epidermis, cells, and cell remnants have been removed. The remaining dermal layer is freeze dried to retain the extracellular architecture and vascular channels.7 Biochemical components in the matrix include collagen, elastin, and proteoglycans. Like the Restore patch, it is packaged dry and is not cross-linked. The patch comes in a variety of thicknesses, which can be used for different surgical situations. TissueMend Soft Tissue Repair matrix (Stryker Orthopaedics, Mahwah, New Jersey) is derived from fetal bovine dermis.7 It is an acellular, non-artificially cross-linked collagen membrane that is one layer thick. Type I and type III collagens are the primary component of the ECM. Tissue-Mend is packaged dry. The Zimmer Collagen Repair Patch (Zimmer, Warsaw, Indiana) is very similar to the Permacol surgical implant.7 Both are acellular sheets of cross-linked porcine dermis. Cellular material, fats, and soluble proteins are removed prior to the material being cross-linked with diisocyanate, which makes it resistant to enzymatic degradation. Derwin and colleagues performed an in vitro study comparing the biochemical, biomechanical, and cellular properties of these patches to each other and to normal tendon.7 Samples of GraftJacket, TissueMend, Restore, and CuffPatch were tested for stiffness and modulus. In addition, hydroxyproline, glycosaminoglycan, and DNA content were quantified. The group found that commercial ECMs required 10 to 30% stretch before the patches started to bear significant load. Once stretched enough, though, each ECM exhibited a stiffer, linear region and an appreciable breaking strength.7 Overall, SIS ECMs (Restore, Cuffpatch) were stiffer than those of dermal origin (GraftJacket, TissueMend) and reached their maximum mechanical properties at lower levels of stretch. At physiological levels of strain for tendon, the biomechanically tested, material properties of the ECMs tested were an order of magnitude less than human RC tendon (Fig. 14–1). In terms of biochemical composition, the ECMs tested had similar amounts of hydroxyproline and chondroitin/dermatan sulfate glycosaminoglycan as fresh tendon.7 Despite being marketed as “acellularized” biomaterials, TissueMend, Restore, and Graftjacket all contained measurable amounts of DNA; only in CuffPatch was DNA content negligible. Figure 14–1 Stress-strain curves of FDS Tendon compared with extracellular matrices. (From Derwin K, Baker A, Spragg K, Leigh D, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair: biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am 2006; 88:2265–2272. Reprinted by permission.) The results of this study support the argument that these patches tend to be more of a biological enhancement to healing as opposed to devices intended to restore mechanical function. Because there are limited clinical studies evaluating the use of ECMs in RC repair, it is difficult to comment on the implications of animal DNA in these patches. Acellularization is performed for three reasons: to reduce antigenicity,8 enhance host cell infiltration with appropriate cells,9 and prevent transmission of infection.12 Case reports of noninfectious edema, following the use of Restore, contend that the reactions are, in part, due to the presence of porcine DNA. However, further studies are needed to determine the clinical implications of incomplete acellularization.13,14 To date, only a few clinical studies evaluate ECMs for RC repair. Small intestine submucosa ECMs have been used successfully in the repair of abdominal walls,13 vascular grafts,14 and bladder reconstruction.15 The orthopedic literature has several studies documenting the enhancement of tendon healing secondary to the use of SIS scaffolds.16–24 Unfortunately, clinical studies have not been as promising. Sclamberg et al19 retrospectively reviewed 11 consecutive patients who underwent open treatment with SIS reinforcement for massive or large RC tears. Patients were evaluated with postoperative MRI at a minimum of 6 months after the index repair and with clinical exam. Re-tears were documented in 10 of 11 patients. Only one repair remained intact per MRI at 10 months postoperatively. There were no statistically significant differences between preoperative and postoperative shoulder scores, and 5 patients scored worse postoperatively. The authors concluded that the use of SIS ECMs for large and massive RC tears is ineffective. A more recent study by Iannotti et al13 echoed these poor results. A randomized, controlled study was performed on 30 patients with large or massive, chronic two-tendon tears. The RCs were treated with open repair using bone tunnels and a combination of modified Mason–Allen and horizontal sutures; and all patients underwent concomitant acromioplasty. Fifteen patients were randomized to a group that underwent repair augmented with the Restore patch. The patch was sewn under tension over the top of the repair from tendon to bone. All patients were evaluated at one year postoperatively with magnetic resonance arthrogram (MRA), PENN shoulder score, and SF-36 questionnaire. Nine of 15 repairs healed in the control group versus four in the augmented group (p = 0.11). When the rate of healing was adjusted for the effect of tear size, repairs done without the Restore patch were 7% more likely to heal. In addition, the median postoperative PENN shoulder score was 83 points in the augmented repair group and 91 in the control group. In this study, 15 more patients would have been needed to show a statistically significant less favorable result with the use of the Restore patch; however, according to the authors, “… there was no reason to continue the protocol… when [they] already had a clear indication that augmentation would not improve the clinical result.”13 The authors concluded that surgical repair with SIS did not improve the rate of tendon healing and did not improve clinical outcome scores. In fact, there was a trend toward less favorable results in patients treated with the ECM.13 The use of these scaffolds is not without complications. In the above referenced study by Iannotti et al, 3 of the 15 patients developed a sterile inflammatory reaction. These manifestations developed between 3 and 4 weeks postoperatively. One patient was treated with irrigation and débridement; one was treated with oral antibiotics until results from a shoulder aspiration came back as negative for infection; and the final patient’s symptoms (erythema and increased skin temperature) resolved without treatment. The final PENN shoulder scores in these patients tended to be among the highest for the augmentation group, indicating that these reactions did effect final outcome.13 Malcarney and colleagues reported on 25 patients undergoing RC repair with a Restore patch augment.14 Four of these patients developed an overt inflammatory reaction at an average of 13 days after surgery. All patients were treated with open irrigation and débridement. As mentioned above, it is possible that these reactions stem from porcine DNA still present in the patch, but further immunologic studies and increased clinical follow up is necessary to better understand the potential complications of these ECMs.
Extracellular Matrix Scaffolds
Clinical Studies of Extracellular Matrices
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree