Abstract
Twenty-five COPD patients, aged 65 years or above, were recruited to test their ability to use dry powder inhaler Handihaler ® (Boeringher-Ingelheim) and Aerolizer ® (Novartis). The results of a score created to evaluate the inhalation technique were compared with age, MMSE, Barthel Index, FEV 1 , maximum inspiratory and expiratory pressures, and peak inspiratory flow (PIF).
Results
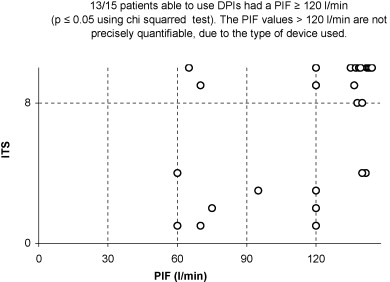
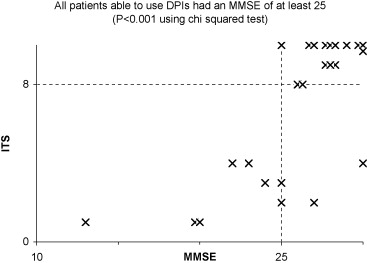
Dry powder inhalers were correctly used by 60% of the patients (15 out of 25). Among the capable ones, 13 out of 15 were aged less than 80 years ( p ≤ 0.02), 13 out of 15 had a maximum inspiratory pressure greater or equal to 53 cm H 2 O ( p ≤ 0.001) and a PIF greater or equal to 120 l/min ( p ≤ 0.05). All skilled patients had a minimum MMSE of 25 ( p ≤ 0.001).
Conclusion
In a geriatric population, age, the decrease of maximum inspiratory pressure and PIF as well as cognitive functions, limit the use of dry powder inhalers.
Résumé
Un collectif de 25 patients, âgés d’au moins 65 ans et atteints de BPCO, a été sélectionné pour déterminer leur aptitude à utiliser les inhalateurs de poudres sèches (IPS) Handihaler ® (Boeringher-Ingelheim) et Aerolizer ® (Novartis). Les résultats d’un score créé pour évaluer la technique d’inhalation (STI) ont été comparés à l’âge, au MMSE, à l’indice de Barthel, au VEMS, aux pressions inspiratoires et expiratoires maximales et au débit inspiratoire de pointe (DIP).
Résultats
Les IPS étaient utilisés correctement par 60 % des patients (15 sur 25). Parmi les patients aptes, 13 patients sur 15 étaient âgés de moins de 80 ans ( p ≤ 0,02), 13 patients sur 15 avaient une pression inspiratoire maximale supérieure ou égale à 53 cm H 2 O ( p ≤ 0,001) et un DIP supérieur ou égal à 120 l/min ( p ≤ 0,05). L’ensemble des patients aptes avait un MMSE minimal de 25 ( p ≤ 0,001).
Conclusion
Dans une population gériatrique, l’âge, la diminution de la pression inspiratoire maximale et du DIP ainsi que l’altération des fonctions cognitives limitent l’utilisation des IPS.
1
English version
1.1
Introduction
The elderly population may have difficulty in correctly using currently available inhaler delivery systems. This can be due to one or more of the following: learning difficulties associated with a lack of cognitive function, impaired motor coordination, insufficient inspiratory flow .
Dry powder inhalers (DPIs) are commonly used to treat COPD. Unlike metered-dose inhalers, there is no need to coordinate the delivery of aerosol drug with an inspiration breath, nor to use of a spacer. Nevertheless, patients need instruction and they have to generate an adequate inspiratory flow rate, specific to each device.
The aim of the study was to investigate the ability of elderly COPD patients to use two types of DPIs.
1.2
Method
This clinical study is comprised of 25 consecutive COPD patients, aged 65 years or above. They were transferred from internal medicine, orthopaedic surgery and traumatology units to the Rehabilitation and Treatment Centre (RTC) of the “Riviera Hospital”. The diagnosis of COPD was either known prior to admission at the RTC or suspected during their stay. In order to meet the inclusion criteria, a spirometry test was conducted in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations , to confirm the diagnosis.
As is often seen in the geriatric population, many patients presented with several comorbidities. Those suffering from acute neurological pathologies (cerebrovascular accident, craniocerebral trauma) or neuromuscular diseases were excluded from the study.
None of the recruited patients had been previously treated with DPIs. They were all informed about the nature, purpose and implications of this study and verbal consent was obtained from all participants.
The study was carried out between February 2004 and September 2005.
The outcome measures included a spirometry test, the maximum respiratory pressures and the peak inspiratory flow (PIF). The inhalation technique and cognitive function were also evaluated.
Spirometry measurements were taken with a portable device “Microlab ML 3500 ®” (MicroMedical Ltd, Chatham; Kent, UK), according to the recommendations of the American Thoracic Society (ATS) .
The oral and nasal pressure measurements were carried out with a MicroMPM ® device (MicroMedical Ltd, Chatham ; Kent, UK) in accordance with ATS recommendations . The oral maximum inspiratory pressure (MIP) was measured from the residual volume (RV) and the maximum expiratory pressure (MEP) was measured from the total lung capacity (TLC).
The sniff nasal inspiratory pressure (SNIP) was measured from the functional residual capacity (FRC) . The highest value of either the MIP or SNIP was employed.
The PIF was measured with an inspiratory flow meter “In-check ® DIAL” (Clement Clarke International Ltd, Harlow, UK) – Fig. 1 . The device has a maximum flow rate of 120 l/min with an accuracy of ± 5 l/min. The mouthpiece setting, providing no resistance, was selected. Patients were seated without support and the manoeuver started from the RV. The best of three values was employed. The DPIs used ( Figs. 2 and 3 ) were Handihaler ® – Spiriva ® – (Boeringher-Ingelheim, Gmbh, Basel, Switzerland) and Aerolizer ® – Foradil ® – (Novartis AG, Basel, Switzerland).


The inhalation technique was assessed by a score (the Inhalation Technique Score or ITS) developed for this study ( Table 1 ). Each step in the procedure was recorded 0 for failure, and 1 for success.
| Score | |
|---|---|
| No idea how to use the inhaler | 0 |
| Vague idea how to use the inhaler (aware of the step-by-step inhalation technique, but numerous trials and errors) | 1 |
| Inhaler completely open | 2 |
| Capsule taken from the pack, placed in receptacle | 3 |
| Perforate the capsule | 4 |
| Breathe out of DPI device | 5 |
| Breathe out fully or as long as possible, away from device | 6 |
| Successful PIF rate (vibration of capsule) | 7 |
| Successful PIF rate (vibration of capsule) and complete inhalation until TLC | 8 |
| Apnea ≤ 5 seconds | 9 |
| Apnea of 10 seconds | 10 |
A chronological sequence of steps was mandatory. An adequate inhalation technique was only accepted if the score was 8/10 or more. All patients were trained once daily, for three consecutive days. Physiotherapists firstly taught the inhalation technique to the patients using an inhaler device with placebo capsules, and patients were then asked to reproduce the entire sequence of events. The same physiotherapist then evaluated the patient’s technique after the third session.
If the score was less than 8, this value was employed as the final score. If the score was 8 or above, the ITS was repeated after one week in order to allocate the final score.
Other components were the body mass index (BMI), the MMSE and the Barthel Index . The data are presented as means and standard deviations. The p value calculated using Student’s t -test was used to evaluate the differences between the mean values, and the Chi-squared test was employed for the threshold values. A value of p ≤ 0.05 was considered significant.
1.3
Results
Twenty-five patients between 65 and 94 years of age were included in this study. Their general baseline values are shown in Table 2 . The results indicate that DPIs Handihaler ® and Aerolizer ® were correctly used by 60% of the patients (15 out of 25). Their data are given in Table 3 . The difference between the two groups with respect to mean age, MMSE and maximum inspiratory pressure (MIP or SNIP) was statistically significant. However, there was no significant difference between mean values of FEV 1, predicted FEV 1 % and the Barthel Index of the two groups.
| All patients | Men | Women | |
|---|---|---|---|
| Number of patients | 25 | 19 | 6 |
| Mean age (years) | 76.5 ± 7.5 | 76.6 ± 8.2 | 76.3 ± 5.2 |
| Mean MMSE | 25.9 ± 3.9 | 25 ± 4.2 | 27 ± 3 |
| Number of patients with MMSE < 25 | 6 | 5 | 1 |
| Number of patients with MMSE ≥ 25 | 19 | 14 | 5 |
| Mean Barthel index | 91 ± 10.2 | 90 ± 11.1 | 95 ± 5.5 |
| Mean FEV1 L | 1.03 ± 0.35 | 1.11 ± 0.34 | 0.79 ± 0.27 |
| Mean FEV1% pred | 44 ± 17 | 45 ± 17.4 | 43 ± 15.2 |
| Mean maximum inspiratory pressure cm H 2 0 | 60 ± 23 | 61 ± 24 | 57 ± 19 |
| Mean maximun expiratory pressure cm H 2 0 | 85 ± 33 | 90 ± 34 | 71 ± 26 |
| Patients with an PIF < 120 l/min | 7 | 5 | 2 |
| Patients with an PIF ≥ 120 l/min | 18 | 14 | 4 |
| Ineffective inhalation Score < 8 | Successful inhalation Score ≥ 8 | P value (Student’s t -test) | |
|---|---|---|---|
| Number of patients | 10 | 15 | |
| Age (years) | 80.3 ± 9.1 | 74 ± 5.1 | ≤ 0.01 |
| MMSE | 22.9 ± 4.6 | 27.9 ± 1.5 | ≤ 0.001 |
| Maximum inspiratory pressure cm H 2 O | 45 ± 10 | 71 ± 23 | ≤ 0.05 |
| PIF ≥ 120 l/min (number of patients) | 5 | 13 | ≤ 0.05 (Chi 2 test) |
| FEV1 L | 1.00 ± 0.39 | 1.05 ± 0.34 | ns |
| FEV1% pred | 49 ± 21 | 42 ± 13 | ns |
| Barthel Index | 88 ± 9.5 | 93 ± 10.5 | ns |
For those patients able to use the DPIs, 13 out of 15 were less than 80 years old ( [CR] ), 13 out of 15 had a MIP of greater or equal to 53 cm H 2 O ( [CR] ) and a PIF greater or equal to 120 l/min ( [CR] ). All able users had a minimum score of 25 on the MMSE ( [CR] ). These results are statistically significant according to the analysis of the sub-groups using the Chi-squared test ( Graphs 1–4 ).


1.4
Discussion
Topical inhaled drugs, delivered by nebulisers or devices such as metered-dose inhalers and DPIs are essential in treating symptomatics COPD patients .
Handling errors are frequently observed, the age, the severity of the ventilatory obstruction as well as the reduction of the PIF playing an unfavourable role .
For example, in a sample of COPD patients with a mean age of 67 years, only 24% used metered-dose inhalers and only 40% used DPIs correctly . The advantages of a DPI (Handihaler ® ) compared with a metered-dose inhaler was corroborated in another study .
In addition, cognitive impairment, more frequently seen among older COPD patients , is more likely to hinder the training process and prevent the correct usage of a DPI. We used the MMSE to assess cognition level among patients. The maximum score of this outcome measure is 30. If the value is less than 24, dementia may be suspected .
In our study, 60% of geriatric patients were able to use the DPIs correctly, after having received preliminary tuition, which was repeated over three consecutive days. However, an age higher than 80 years, a MMSE less than 25, a MIP less than 53 cm H 2 O and a PIF less than 120 l/min were generally associated with insufficient ITS values. On the other hand, the severity of the COPD did not constitute a limiting factor for the DPIs used (Handihaler ® , Aerolizer ® ).
The ITS values were determined using a chronological, step-by-step procedure comprised of various elements to assess the correct use of DPIs. With a score of at least 8/10, the patient is able to inhale with a sufficient flow and volume to ensure an efficient therapeutic inhalation. The ITS can be used for the initial training of patients and for successive trials, required for a rigourus follow-up. In addition, the ITS is transferable from one device to another.
In both DPIs, an adequate inspiratory flow rate was monitored audibly by the vibration of the capsule in the device. All the patients were able to generate this vibration, indicating an adequate inspiratory flow to micronise the dry powder, irrespective of their inspiratory pressure and PIF values.
Ultimately, all patient failures were due to a deficit in motor function or impaired comprehension. This emphasizes the importance of evaluating cognitive performance among the elderly suffering from COPD.
For those patients who are unable to manage a DPI correctly, an alternative solution is to use nebulisers or metered-dose inhalers coupled with a spacer and the assistance of a third person .
In conclusion, age and cognitive impairment are the principal elements restricting the correct use of the DPIs among the geriatric population.
2
Version française
2.1
Introduction
L’utilisation adéquate des différents systèmes d’inhalation est difficile pour les patients âgés. Cela peut être dû aux difficultés d’apprentissage liées à l’altération des fonctions cognitives, aux troubles de la coordination motrice et à la difficulté d’adapter les paramètres ventilatoires aux besoins de l’inhalation .
Les inhalateurs de poudres sèches (IPS) sont fréquemment employés pour le traitement de la BPCO. Contrairement aux aérosols-doseurs, ils ne requièrent pas de coordination entre le déclenchement de la cartouche d’aérosol et l’inspiration, ni l’emploi d’une chambre d’inhalation. Les dispositifs utilisés nécessitent toutefois un apprentissage et un débit inspiratoire adapté à chaque appareil.
Le but de cette étude était d’évaluer la capacité d’utilisation de deux dispositifs d’inhalation de poudres sèches chez des patients âgés atteints de BPCO.
2.2
Méthode
Il s’agit d’une étude clinique portant sur 25 patients consécutifs atteints de BPCO, âgés d’au moins 65 ans ; transférés au Centre de traitement et de réadaptation (CTR) de l’hôpital Riviera en provenance des services de médecine interne, de chirurgie orthopédique ou de traumatologie. Le diagnostic de BPCO était connu lors de l’admission au CTR ou suspecté durant le séjour. Avant l’inclusion, une spirométrie était effectuée pour confirmation du diagnostic selon les recommandations émises par le Global Initiative of Chronic Lung Disease (GOLD) .
Comme fréquemment en gériatrie, les patients présentaient souvent plusieurs comorbidités. Seuls ceux souffrant de pathologies neurologiques aiguës (accident vasculaire cérébral, traumatisme craniocérébral) ou d’atteintes neuromusculaires étaient exclus de l’étude.
Aucun des patients sélectionnés n’avait été préalablement traité avec des poudres sèches.
Tous les patients étaient informés du propos, des caractéristiques et de la nature de cette étude et avaient fait part oralement de leur consentement.
Les observations ont été réalisées entre février 2004 et septembre 2005.
Les investigations comprenaient une spirométrie, la mesure des pressions respiratoires et du débit inspiratoire de pointe (DIP). La technique d’inhalation ainsi que les fonctions cognitives étaient évaluées.
Les spirométries étaient effectuées avec un appareil portatif muni d’un dispositif à turbine de modèle « Microlab ML 3500 ® » (MicroMedical Ltd, Chatham ; Kent, Royaume-Uni) selon les recommandations de l’American Thoracic Society (ATS) .
La mesure des pressions buccales et nasales était réalisée à l’aide de l’appareil MicroMPM ® (MicroMedical Ltd, Chatham ; Kent, Royaume-Uni) selon les recommandations de l’ATS . La pression inspiratoire buccale maximale (PImax) était mesurée à partir du volume résiduel (VR). La pression expiratoire maximale était mesurée à partir de la capacité pulmonaire totale (CPT). La sniff nasal inspiratory pressure (SNIP) était mesurée à partir de la capacité résiduelle fonctionnelle (CRF) .
La valeur la plus élevée de PImax ou de SNIP était retenue.
Le DIP était mesuré avec le débitmètre de type « In-check ® DIAL» (Clement Clarke International Ltd, Harlow, Royaume-Uni) – ( Fig. 1 ). La précision de la mesure est de ± 5 l/min et le maximum mesurable est de 120 l/min. L’orifice prévu pour mesurer le DIP sans résistance était utilisé. La mesure du DIP était effectuée en position assise sans appui à partir du VR. La meilleure des trois valeurs était retenue.










