CHAPTER 15
The Role of Physical and Occupational Therapy in the Evaluation and Management of Spasticity
Susan Reeves and Kelly Lambeth
Spasticity is a common complication of neurologic insults, such as stroke and spinal cord injury (SCI). It is associated with additional signs and symptoms of upper motor neuron (UMN) damage, including paresis, loss of selective control, and spastic cocontraction. These factors can lead to impairment and significant functional limitations (1). The goal of spasticity management is to reduce hypertonia while considering the impact of spasticity on function and overall well-being of the patient (1). Spasticity may be focal or multifocal. Combined with other symptoms of UMN insult, problems are highly variable, necessitating individualized, goal-directed treatment (2). Although there are few comparative studies demonstrating the efficacy of one therapeutic approach over another, experienced spasticity management teams propose that optimal spasticity management involves coordinated medical management (pharmacological and surgical interventions) combined with rehabilitation efforts (1), most frequently delivered by physical and occupational therapists.
This chapter explores the role of physical therapy (PT) and occupational therapy (OT) in the multidisciplinary management of spasticity. Physical and occupational therapists participate in the clinical assessment of spasticity, individualized goal setting for spasticity management, and delivery of rehabilitation interventions. This assessment includes appreciation and quantification of both the positive and negative aspects of spasticity, consideration of the presence of other UMN symptoms, and impact of these factors on overall functional abilities and goal attainment. Rehabilitation goals for patients undergoing spasticity intervention include: (a) improvement of functional alignment, (b) improvement of selective motor control (SMC), (c) improvement of strength, (d) improvement of functional abilities, and (e) enhancement of community participation.
FACTORS TO CONSIDER
When determining the optimal course of management for a patient with spasticity, the therapist must weigh the many aspects that can influence the evaluation and treatment decision process. These include, but are not limited to, benefits versus detriments of spasticity; timing of medical interventions; distribution (focal, multifocal, generalized); age of the patient; prognosis; time since onset; medical condition; and underlying etiology of the UMN damage.
The deleterious effects of spasticity, especially when impeding function, are considered. Spasticity can interfere with bed mobility, transfers, wheelchair mobility, ambulation, eating, toileting, hygiene, sexual function, and dressing (3–6). Not only can spasticity impair functional mobility, it can also be extremely painful. The pain and spasm cycle can become debilitating to the person emotionally, leading to severe depression and further isolation (7,8). When left untreated, spasticity can lead to shortening of the muscles and tendons and ultimately to the development of joint contractures (7,9,10). These secondary consequences of spasticity (soft tissue hypoextensibility, contracture, bony deformity) may further limit the patient’s ability to participate in rehabilitation and result in the need for additional medical intervention (orthopedic surgery, casting, bracing). Additionally, motor impairment due to paresis may be further aggravated by muscle and joint contracture and changes in muscle contractile properties caused by immobilization (11). Based on the problems that can result from spasticity, primary and secondary consequences, the need to treat spasticity early and aggressively is advocated by many teams.
Although spasticity can be devastating to the overall function of the patient, there are also potential benefits of the condition (12). Spasticity can assist with maintaining muscle tone and bulk over bony areas that are prone to pressure and skin breakdown, such as ischial tuberosities and sacrum, and potentially mitigate the condition. In addition, the muscle pumping action that results from muscle overactivity can potentially aid in overall circulation and reduce the risk of deep vein thrombosis. Increases in spasticity in a person with SCI and other neurologic conditions can serve as a red flag and warn of urinary tract infection, skin breakdown (7), development of heterotopic ossification (13), or syringomyelia (14). Spasticity can facilitate function. Increased muscle tone can assist in the performance of transfers, bed mobility, standing, ambulation, sexual function, and bladder management (15). The clinical team must weigh the loss of the positive effects of muscle overactivity against the benefits of treatment when making management decisions. The patient’s primary goal for spasticity reduction must be considered in the multidisciplinary management of spasticity.
Spasticity is common in children with cerebral palsy (CP) and affects physical development. Spasticity affects muscle growth and joint alignment and may increase with age. For this reason, the growth and development trajectory for children requiring spasticity management must be considered in the timing of interventions. In children, UMN injury may result from a static injury to the central nervous system (CNS); however, the clinical picture that results is described as “nonprogressive but ever-changing” (16). When treating children, the fact that significant tone can place stresses on bones and joints, which can in turn lead to deformity, must enter into the decision process and commonly results in more aggressive early intervention. It is believed that eliminating spasticity enables the child to develop SMC more effectively and functionally (17) and potentially reduces or postpones the need for more aggressive management for orthopedic deformity (17).
Developing patient-specific treatment goals in rehabilitation is crucial. Examples of potential targeted goals include, but are certainly not limited to, maintaining or improving the patient’s overall functional mobility and independence, alleviating pain, improving energy expenditure of movement, preventing or limiting contracture development, maintaining skin integrity, improving positioning and overall functioning with wheelchair management, bracing and other positioning equipment, and decreasing the level of assistance required by caregivers. The setting of unrealistically high goals can be every bit as much of a problem as choosing ones that are too low. It is important to develop a plan of care that is tailored to the individual, has buy-in from the patient and caregivers, and is well integrated with the overall medical management.
CLINICAL EVALUATION
The evaluation of individuals with spasticity requires a multidisciplinary approach, including input from physicians; physical, occupational and speech therapists; nurses; and most importantly the patient and his or her caregivers. A thorough assessment incorporating all of the factors discussed earlier is required for the development of the optimal treatment plan. A vital component of the evaluation process is a standard neuromuscular evaluation including range of motion (ROM), strength, posture, skin integrity, coordination, balance, transfers, sensation, cranial nerve testing, functional mobility, gait, and reflexes. Additionally, nutritional status and overall levels of activity are key factors.
Spasticity, abnormal reflexes, muscle spasms clonus, and dyssynergic movement patterns have been described as the “positive” features of UMN syndrome (UMNS). The “negative” features include weakness, loss of dexterity, and fatigability (18). Both positive and negative components of UMNS contribute to the functional deficits that are seen clinically, and evaluation for their presence must be part of the patient assessment. Perhaps, the most frequently cited definition comes from Lance (19), who defined spasticity as “a motor disorder characterized by a velocity dependent increase in tonic stretch reflex (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome.” The term spasticity has become commonly used almost in a more generic sense to include all the other components of the UMNS, including hyperreflexia, clonus, clasp-knife rigidity, exaggerated cutaneous reflexes, cocontractions, dystonia, and associated reactions that occur as a result of positional changes or noxious stimuli (10,20). Pandyan et al (21) described spasticity as more than a pure motor disorder that does not result solely from the hyperexcitability of the stretch reflex. Instead, it should be defined as “disordered sensori-motor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” (21). This definition is more broad than Lance’s because it includes not only the velocity-dependent spasticity produced as a joint is rapidly flexed or extended at a single point in time but also the increase in tone and muscle spasms witnessed with positional and postural changes during phases of gait and with other functional movements. Spasticity is not just an isolated event to be assessed only at a single point in time but instead is a dynamic event that requires a functional-based assessment to fully appreciate the impact it has on the overall function.
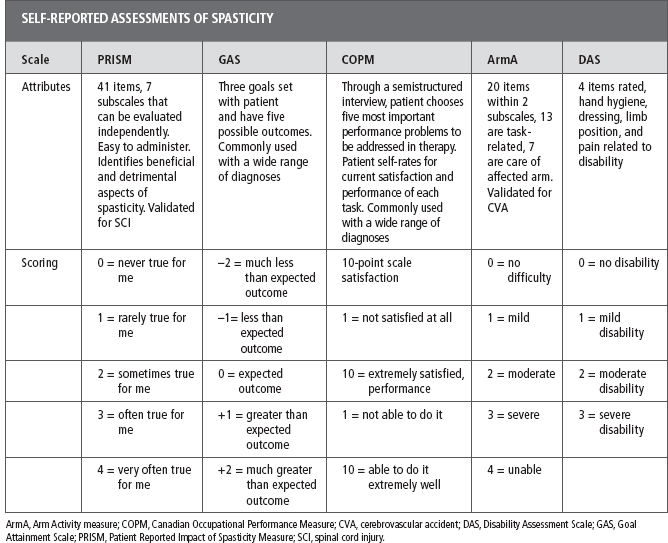
Valid and reliable metrics are required to evaluate functional changes and treatment efficacy. Numerous outcome metrics are available for this purpose; an extensive discussion of this topic can be found elsewhere in this book. To facilitate a better understanding of the authors’ comments, some of the scales most commonly used by therapists are briefly discussed. The authors have placed these scales into three categories based on the data collected to obtain the score: physical assessment of spasticity, self-reported assessments to assist in the overall evaluation, and treatment framework (Tables 15.1 and 15.2). For the therapist treating the patient, it is important to not only quantify the severity of spasticity but also to evaluate its effect on function and goal attainment. Comprehensive spasticity management programs aim to facilitate individualized patient-specific goal attainment. Thus, research, clinical trials, and evaluation tools must include the patient’s self-report and experience to supplement the current battery of measures (22). Physical and occupational therapists participate in the ongoing assessment of spasticity to evaluate progress toward goals and also to assist with making recommendations for modifications to the current plan of care and/or addition of complementary interventions.
TABLE 15.1
ASHWORTH AND MODIFIED ASHWORTH SCALE | |
AS | MAS |
0 (1) = No increase in tone | 0 = No increase in tone |
1 (2) = Slight increase in tone, giving a catch when the affected part is moved in flexion or extension | 1 = Slight increase in tone, manifested by a catch and release, or by minimal resistance at end ROM when the affected limb is flexed or extended |
2 (3) = More marked increase in tone but the affected part is easily flexed | (1+) = Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM |
3 (4) = Considerable increase in tone, passive movement is difficult | 2 = More marked increase in muscle tone through most of the ROM, but the affected part is easily moved |
4 (5) = Affected part is rigid in flexion or extension | 3 = Considerable increase in muscle tone, passive movement is difficult |
4 = Affected joint is rigid in flexion or extension | |
AS, Ashworth Scale; MAS, Modified Ashworth Scale; ROM, range of motion.
Physical Assessments
Physical assessments of spasticity often involve moving the affected joint rapidly through its available ROM and quantifying the severity of the tonal response. Commonly used manual methods for evaluating spasticity include the Ashworth Scale (AS) and the Modified AS (MAS), which are discussed in Table 15.1. There are numerous other measures, including the Tardieu Scale (TS) and Modified TS (MTS), which are also discussed.
The AS and MAS are easy to perform and require no specialized equipment to complete; however the score obtained is subjective. The validity, interrater reliability, and correlation to other measures of spasticity and function are inconsistent and often questioned as useful tools in the literature (12,18,23–26) particularly when assessing the lower extremity (27). In contrast, Brashear et al (28) found good interrater and intrarater reliability when assessing the spasticity of the wrist, fingers, and elbow in persons who have had a stroke. Likewise, Sköld et al (29) found significant correlation between the MAS and the patient-reported spasticity using a Visual Analog Scale (VAS) in 45 persons with SCI.
TABLE 15.2
The AS and the MAS are subjective in nature. They do not have standardized procedures for patient positioning, scoring, or overall test implementation (25). Ideally, the clinician should choose a position that is most comfortable for the patient and gives the best picture of the overall spasticity. It is important that the patient be tested in the same position during subsequent sessions to optimize reliability. The affected joint is passively moved through its available ROM at a speed sufficient to induce a spastic response. This presents a major limitation to both scales because the speed of movement has not been well established.
Multiple studies report that 1 s may be the best time to take a joint through its available ROM (8). The resistance to movement, where it occurs in the ROM and the strength of the resistance are noted and quantified. The AS uses a 5-point 0 to 4 scale, whereas the MAS adds 1+ to the scale as well (Table 15.1 [30]).
The AS was originally designed to evaluate the antispasmodic effects of carisporodol in multiple sclerosis (MS [31]). Both scales have subsequently been used with a variety of etiologies including brain injury (25), stroke (28), and SCI (12). The fact that spasticity differs based on etiologies may account for some of the variability in the literature that has examined the MAS and AS (8,12). The additional factors to consider when administering either scale include the time of day, emotional status, current health issues, repeated stretching or ROM before the testing period, pain, and fatigue (8). Any of these factors can either lead to an increase or decrease in spasticity, thereby giving the tester a skewed result. In addition, it is important to note that the AS and MAS only evaluate the velocity-dependent nature of spasticity across a single joint (12) at a single point in time. Although both scales have been weakly associated with quality of arm skills, gait velocity, stride length, and gross motor functions (25), there have been limited data demonstrating a significant association between the AS and any specific function.
The AS and MAS attempt to measure resistance to passive movement using one speed of movement. It is argued that the velocity-dependent nature of spasticity is not truly assessed and that these measures evaluate a combination of tissue contracture, spastic dystonia, and spasticity (34). For these reasons, the TS and MTS have gained favor as more accurate and valid measures of spasticity following Lance’s definition. The TS and MTS are similar scales based on the work of Guy Tardieu who created a clinical method to measure spasticity by comparing threshold angles of muscle reaction to stretches at several predefined speeds (32,33). The MTS and TS have been modified into more clinically useful tools. The scales rate spasticity as the difference between reaction to stretch at the fastest and slowest possible stretch velocities deemed by the examiner. The slow velocity stretch remains below threshold for any significant stretch reflex and provides a measure of the passive ROM. In contrast, the fast velocity stretch maximizes the stretch reflex and attempts to quantify the point in the ROM at which the spastic catch is elicited. The difference between the angle of catch and the angle of passive motion is noted and suggested as a measure of spasticity. Clonus, fatigable or sustained, is also noted (34). Reliability of the MTS and TS has been evaluated with initial reliability for assessing spasticity in CP promising (34). However, there is need to further establish the reliability and validity for the TS and MTS in CP and across other population of individual with UMN insult (35).
Patient-Reported Assessments
A critical component to the overall evaluation and management of spasticity is the feedback from the patient and his or her caregivers. The negative and positive consequences of UMN damage are highly variable and necessitate individualized evaluation, treatment, and goal setting. Additionally, symptoms can fluctuate based on the many factors described earlier. The patient’s self-assessment can provide therapists with a clearer picture of the overall nature of spasticity and the impairment and functional deficits that it evokes. This information facilitates the development of an individualized treatment plan. Research has shown that clinical examination does not always elicit spasticity in patients who report it and that examination of one or more symptoms of spasticity does not correlate with the person’s self-report of spasticity, with function, or with each other (36,37). Therefore, it is crucial that the therapist take into account the patient’s self-report of symptoms and effect on his or her function (15). The Patient Reported Impact of Spasticity Measure (PRISM), the Goal Attainment Scale (GAS), Canadian Occupational Performance Measure (COPM), the Arm Activity Measure (ArmA), the Barthel Index, and the Disability Assessment Scale (DAS) are valuable self-report tools.
The PRISM is a self-report evaluation tool created to measure both positive and negative effects of spasticity in patients with SCI (38). The self-report consists of 41 questions related to everyday situations and how spasticity affects the patient in completing these tasks. The items are organized into seven subscales: (a) impact on activities of daily living (ADLs), (b) social avoidance, (c) psychological impact, (d) impact on health service utilization, (e) impact on independence, (f) embarrassment, and (g) positive impact of spasticity. Examples of the items are “Kept me from going out with friends”; “Helped me or my attendant change my position”; and “Kept me from working or doing household tasks as much as I wanted to.” The items are rated on a 0 to 4 scale with (0 = “never true for me” and 4 = “very often true for me”). This tool has the ability to capture information from the patient related to not only functional tasks but also from the psychological impact that impairs interaction with others ranging from community access to intimate relationships. The GAS uses goals established by the patient and therapist. Each goal consists of five possible levels of attainment that range from -2 to +2: -2, “much less than expected”; –1, “less than expected outcome”; 0, “expected outcome”; +1, “greater than expected outcome”; and +2, “much greater than expected outcome.” Ashford and Turner-Stokes (39) recommended three goals to be established to maximize the potential benefits of increased clarity and increased patient satisfaction and cooperation. Fewer goals can be used if time and resources are limited. Turner-Stokes et al (40) found that in assessing outcomes of focal spasticity management, the GAS assisted in identifying patient- and caregiver-specific goals that would not have been established using standardized measures.
The DAS is a four-item self-report/observation tool that primarily addresses upper extremity (UE) functional difficulties and patient’s rate of perceived difficulty in the areas of hand hygiene, including washing hands, cutting nails, and dressing, which includes putting on gloves, limb position, and pain. Items are rated on a scale from 0 to 3: 0, no disability; 1, mild disability (noticeable but does not interfere significantly with normal activities); 2, moderate disability (normal activities require increased effort and/or assistance) and 3, severe disability (normal activities limited). The test is short and best conducted through administration by a therapist with the patient. One limitation is that a patient who has incorporated adaptive strategies to compensate for a hemiplegic extremity may rate himself or herself lower (less disability) than actual performance (28).
The COPM is a client-centered outcome tool that measures a patient’s self-perception of his or her most meaningful everyday tasks. The therapist uses a semi-structured interview to help the patient identify the most important performance problems. Each time the tool is administered the same tasks are rated by the patient on a 10-point scale where 1 indicates poor performance and low satisfaction, and 10 indicates very good performance and high satisfaction. When reevaluating, the therapist asks the client to self-rate performance and satisfaction for the same performance issues. The change in scores is calculated to measure improvement. Although this assessment can take more time than some of the others to administer, it has the opportunity to provide truly patient-centered goals that are specific to the spasticity intervention (41).
The ArmA is a self-rated outcome measure with two subscales to total 13 items. Each subscale is scored separately with the patient determining how much difficulty he or she has had completing each task in the last 7 days. The patient and/or caregiver rates each task on a scale from 0 to 4 with 0 = no difficulty and 4 = unable to do activity. One benefit to this evaluation is the limited number of items, which include both active and passive activities and both distal and proximal performance of the UE during ADLs. Examples of these evaluated items are cleaning the hand, cleaning the armpit, eating with knife and fork, and using a key to unlock a door. This evaluation can help with functional goal-setting and involving not only the patient but also the caregivers (42).
The Barthel Index is an outcome measure comprised of 10 items that measure global disability and function. Evaluated items are self-care and activities active in nature including dressing, bathing, feeding, gait, and stair mobility. Each item is scored from 0 to 10 or 0 to 15: 0 = unable to do task and 10 or 15 (whichever is the highest possible score for the item) = independent. A middle score would indicate that the patient is completing more than 50% of the task. Scores are obtained by recording what the patient actually does, not what the patient could do. Based on observation within the previous 24 to 48 hours, the Barthel establishes a measure of how much assistance a patient needs (43).
Functional Assessments
Objective assessment of active function is invaluable in the evaluation, goal setting, and ongoing measurement of progress toward treatment goals. A number of scales have been developed to measure upper limb function in various populations and settings (44). Task-specific assessment tools for upper limb function most commonly cited for this population are the Action Research Arm Test (ARAT), the Wolf Motor Function Test (WMFT), and the Modified Frenchay. Walking function assessed by gait speed is proposed as the most meaningful objective assessment of active lower limb function for patients undergoing spasticity treatment (44).
The ARAT includes four subtests in the areas of grasp (six items), grip (four items), pinch (six items), and gross movement (three items). Scoring is based on the total score calculated from individual scores for each task. Scores range from 0, “cannot perform task”; 1, “can partially perform the task”; 2, “can complete the task but took abnormally long or had great difficulty”; 3, “can perform normally.” Maximum scores for each category: grasp, 18; grip, 12; pinch, 18; and gross movement, 9; for a total possible score of 57 (45). The Modified Frenchay Scale (MFS) is a task performance assessment in which the patient completes a series of bimanual and unilateral tasks while a clinician observes and scores the patient’s performance either in real time or through recorded video. The MFS includes 10 everyday tasks such as picking up a water bottle, applying toothpaste to toothbrush, simulated cutting with fork and knife, and opening and closing a jam jar. The performance of each task is rated with 10-point intervals. Although it is feasible to complete with a single rater, the subjectivity of the scoring questions the validity. The MFS has potential to be used more frequently in the clinical setting if scoring can be objectively measured and interrater reliability established (44).
Timed performance of repetitive reaching has been described as a useful functional outcome measure. If the stretch reflex is inhibited then greater ROM should be achievable and in turn lead to greater function. In the clinic, one can observe this during functional tasks such as reaching to don pants or folding laundry bimanually.
Tests to consider when testing distal control of the arm are the 9-Hole Peg Test, Box and Blocks Test, and the Jebsen Taylor Hand Function Test.
The rehabilitation professional must use good clinical reasoning when choosing the best outcome measures when evaluating a patient with spasticity. For example, which tests are appropriate and how much time will they take to administer? The ARAT and WMFT have been shown to strongly correlate with each other at 14 days and 1, 3, and 6 months. The ARAT takes 10 to 15 minutes to administer and the WMFT takes 30 minutes (45). What are the goals of the patient and caregiver (COPM, GAS)? The examiner must question what will be the best predictor of change related to the goal. Is the goal more passive in nature (GAS, DAS)? Is the goal more active in nature (ARMA, MAL, ARAT, WMFT, fine motor test)?
Tests of walking function are used commonly in clinical practice and clinical research to measure lower limb function. The value and validity of gait speed as a measure of active lower limb function in spastic paresis is well established (44). Walking speed correlates with most kinematic measures of gait in spastic paresis (44) and can be quantified in a number of ways. The 10-m walk test is validated in populations of individuals with spasticity and is a clinically useful tool. Walking speed measured with a stopwatch has high reliability and good concurrent validity compared to timing gate measures. Walking speed and step length have excellent test–retest reliability at 1 week in chronic hemiparesis. In addition to gait speed, step length and cadence can be captured during a timed walking test, as well as physiologic cost index (speed divided by the difference between the heart rate before and after the effort [44]). These measures are clinically useful in assessing the impact of the reduction of hypertonia on the quality and efficiency of movement. The quality of gait-walking movement can be more formally assessed using instrumented gait analysis; however, this tool is not accessible to many clinicians. Clinically available video recording is also useful and is perhaps more practical for objectifying quality markers of gait. Tests of stair climbing and uneven ground walking performance may also be useful tools to evaluate gait function, particularly to reveal quality gait abnormalities associated with spasticity that may be less apparent on level overground walking (44). Evaluating the effectiveness of spasticity interventions and rehabilitation programs is a multiple step process incorporating physical assessments at the impairment level, combined with self-report and physical function tests. Patient and caregiver feedback relative to interventions and progress is key. Formal assessments of progress toward goal attainment (GAS, COPM) have been invaluable in assessing the effectiveness of goal attainment.
REHABILITATION GOALS
The primary rehabilitation goals for patients undergoing spasticity management are to (a) restore functional alignment, (b) improve SMC, (c) improve strength, (d) improve functional performance, and (e) facilitate activity and community participation.
Goal: Restore Functional Alignment
As discussed earlier, UMN damage often leads to paresis and muscle overactivity. Spasticity and disuse from paresis result in tissue immobilization in a shortened position. Immobilization can lead to soft tissue changes within hours (44). Muscle plasticity during this time leads to tissue contracture, involving (a) physical shortening of muscles, tendons, ligaments, joint capsules, nerve vessels, and skin; (b) loss of tissue extensibility; (c) loss of tissue mass; and (d) changes in the contractile properties of muscle tissue (44). Patients undergoing medical therapies to reduce muscle hypertonia benefit greatly from concomitant therapy interventions to restore tissue length and functional alignment of joints. The goal of restoring functional alignment is to facilitate improved performance of functional tasks in the most energy efficient and pain-free manner possible. Rehabilitation interventions most commonly used to restore functional alignment in spasticity management include stretching (manual, static, static progressive, dynamic) and positioning.
Manual Stretching. The exercises and protocols that fall in the category of stretching are some of the primary intervention strategies used by both physical and occupational therapists in the management of patients with spasticity. Stretching is defined as the process of producing elongation. As an intervention, it is commonly used to address numerous other impairments other than spasticity, such as limitations in ROM and functional mobility.
There are numerous methods of applying the modality of stretching, but historically, it is provided by clinicians in a hands-on manual fashion. Manual stretching techniques are heavily used as an adjunct to other therapeutic interventions but are very difficult to standardize and objectify. This has complicated efforts to scientifically study and develop evidence-based practice (EBP). Mechanical devices such as the dynamometer (Cybex) or robotic devices are also used to deliver stretch. In contrast to manual stretch, the use of these devices increases the clinician’s ability to be more objective, allows better standardization for clinical treatment and research protocols, and may better facilitate the creation of guidelines for EBP. On the other hand, these devices can be both extremely expensive and inaccessible to many clinicians, particularly in smaller practice settings. This may force a choice between treatments that can be well controlled and objectively measured but functionally irrelevant versus treatment that is functionally relevant and practical but with a very limited means of standardizing and quantifying (47). The effects of manual stretching on spasticity are limited, with evidence showing no long-lasting changes on spasticity or the underlying etiology. Additionally, a comprehensive review of the evidence suggests that manual stretching is ineffective in contracture management in children with CP (48). However, the detrimental effects of limb immobilization on the passive and active muscle properties are also well established (11). It follows, therefore, that programs that encourage limb mobilization and passive stretch, especially when combined with education to families and caregivers, might assist with reducing the risk of contracture and permanent muscle shortening over time. This in turn can potentially minimize the secondary complications and the need for additional interventions, such as surgical tendon-lengthening procedures. It is important to note that stretching as an intervention is not without risks. Patients have experienced numerous problems as a result of aggressive ROM activities. At times, a clinician can create the very problem that he or she seeks to avoid as a result of providing painful and noxious stimuli. Although rare, avulsions and fractures have been reported in the literature (49). These complications arise often in the presence of other chronic impairments associated with the underlying pathology. Overall, evidence supports stretching for longer intervals of time (10 minutes, 30 minutes) to prevent or reverse soft tissue changes associated with immobility and to reduce spasticity (50,51) and combining stretching with additional modalities such as strengthening exercises (52) to achieve optimal results. Splints, braces, serial casts, and positioning devices are intervention strategies employed to deliver this modality for durations not practical with hands-on stretching. Evidence supporting these interventions is explored in the following.
Static Stretching: Splinting. Static splinting is the use of a removable device worn for a specific period of time to maintain a specific position of the limb to support weak or ineffective joints or muscles (53). Physical and occupational therapists use splints for several clinical indications such as “to increase function, prevent deformity, substitute for lost motion, protect healing structures, maintain ROM, stabilize joints, restrict motion, allow tissue remodeling, improve muscle balance, control inflammation, protect normal structures, decrease pain, strengthen weak muscles, reduce spasticity, and increase patient independence” (51).
Many therapists prescribe reflex inhibitory splinting (RIS) for patients demonstrating spasticity and hypertonicity of the hand and, commonly, the elbow (51). Therapists traditionally operate on the theory that a dorsal-based hand splint is better than a volar-based hand splint because sensory stimulation on the palmar side of the hand would trigger spasticity. Another technique commonly used by therapists is the “functional hand” position with the wrist in 20° to 30° of wrist extension or the antispasticity ball splint with wrist in neutral and fingers abducted and flexed slightly as if resting on a ball. Lannin and Ada (51) reviewed the literature for high-quality randomized controlled trials (RCTs) and found only two high-quality RCTs, one compared splinting up to 22 hours a day and with the wrist in neutral or extended wrist, and one study compared wearing a splint with finger spreader position for different amounts of time ranging from 6 to 22 hours. Two RCTs compared volar and dorsal splinting to see which was better in decreasing hypertonicity (51). The studies reviewed were unable to show that there was a significant decrease in hypertonicity from the splinting related to length of time, position of wrist, or if the splint was dorsal or volar.
Lannin and Ada (51) reviewed three RCTs examining the benefits of hand splinting to prevent contracture after stroke. None of the studies were able to show a significant change in contracture or ROM with variables such as wrist in neutral or extension and length of time the splint was worn. It is of note that none of the studies placed the patient in full stretch of the wrist and hand. Lannin and Ada (51) also concluded that there was no difference in using a splint versus other approaches to preventing contracture.
When a therapist chooses to fabricate a splint or fit for a prefabricated splint, the opportunity for adjustments during the treatment of spasticity should be strongly considered. One such splint on the market is the Saebo Stretch splint (Figure 15.1), a volar hand splint that is made of metal with a soft covering that can be bent to accommodate different wrist and thumb positions.
The splint also has a dynamic component for the fingers with a choice of three hand plates that are able to vary the amounts of resistance for fluctuating spasticity and hypertonicity. The splint can be modified with more or less wrist extension as the patient has changes in hypertonicity, spasticity, and available ROM.
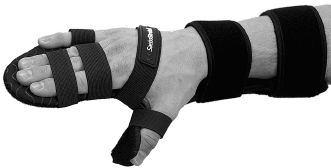
FIGURE 15.1 Saebo Stretch splint.
The current evidence casts concern over the current standard practice of hand splinting. Research has not shown that all-day or all-night splints in addition to usual therapy have any effect on preventing contracture even with changes of wrist position.
A lack of consensus about the design, wearing duration, and wearing compliance of splints continues. There are also continuing questions on how or if splints aid in the recovery process
Hand splinting for spasticity and UMN symptoms would benefit from further RCT research with large sample sizes and homogeneous populations with reliable and accurate measurements. As health care continues to change, therapists will need to be ever more mindful of providing the best care while using resources wisely. One should carefully assess the benefits of each possible intervention with the desired outcome.
Static Progressive Stretching. Static progressive stretching is the use of biomechanical stress relaxation to restore ROM in joint contractures. Techniques used are serial casting (SC) and progressive static splinting.
Serial Casting
The use of serial casting (SC), in the management of spasticity has been used for decades and is typically done after specialized training. It was first described in the 1960s in individuals with CP (54,55). Since then, casting has evolved to include treatment in multiple CNS disorders, including brain injury, SCI, stroke, and MS.
As hypertonia and spasticity can cause a muscle to remain in a shortened position for prolonged periods of time, secondary complications often seen are contractures. Contracture often leads to limitations and impairments in functional mobility with walking, dressing, and ADLs. Pain can accompany the contracture adding to the difficulty of everyday living. A primary indication for SC is in minimizing these secondary complications. Typically, the elbow, wrist, finger, and ankle joints are most commonly treated with SC. Currently, there is no evidence-based clinical guideline for SC patients with CNS disorders. SC is performed based on clinical opinion and historical practice than it is scientific findings (54,55). This is not to say that it is not an effective treatment. Too often, SC is recommended and used once contractures and limitations are present. It is generally felt that a more proactive approach is ideal, and the patients who are deemed at risk should be identified early and treated.
SC involves the stepwise application of a cast made of plaster or fiberglass tape applied circumferentially around an immobilized limb in a desired fixed position. The repeated application of casts with the joint being stretched further with each application leads to improved ROM, increased function, and/or decreased pain (54). SC is discontinued when a plateau is reached and no increase in ROM is noted. At this point, the final cast is bivalved to serve as maintenance orthosis (Figures 15.2–15.4). A bivalved cast is believed to have a better stretch effect with greater torque than a hand splint (51).
The proposed theories suggest that SC reduces spasticity and hypertonia, but the underlying mechanism is largely unknown. However, numerous theories do exist. The neurophysiological theory proposes that casts minimize changes in muscle length, which in turn reduce excitatory input through afferent receptors in the muscle spindles, which in turn reduce reflexive alpha motor neuron excitability. The increased tension on the spastic muscle also results in increased stimulation of the Golgi tendon organs, which inhibits the alpha motor neurons through type Ib afferent fibers (54). Another proposed component is that the circumferential pressure around the spastic muscle and joint provided by the cast is thought to provide neural warmth and reduce cutaneous sensory input (54), which reduces the overall level of interneuron and motor neuron excitability, thus decreasing spasticity (56). A similar effect has been seen with air splints applying circumferential pressure (57). The last proposed theory is the motor learning rationale that casts can provide immobilization to proximal joints so that the distal musculature can gain strength and control. An example of this theory is casting the wrist in extension and thumb in opposition while the fingers are free to complete grasp and release.
A mechanical explanation has also been given as an explanation for the efficacy of SC. When a cast is applied, it provides a stretch of load and long duration, which helps to prevent and correct joint contractures (58,59). Alterations in the mechanical properties of the muscle and tendon itself have been exhibited through casting studies, with animal studies showing an increase in the number of sarcomeres in series in response to casting (60).

FIGURE 15.2 Material used in SC including cast padding, foam padding, fiberglass casting material, and stockinette (cutout foam to protect bony prominences).
SC, serial casting.

FIGURE 15.3 Application of serial cast to treat ankle plantar flexor contractures and muscle overactivity. The clinician is wrapping high on the calf to maximize the mechanical advantage of the cast to provide optimal stretch.
Wearing schedules. As mentioned earlier, serial casts are applied in a sequential and stepwise manner to progressively increase gains in ROM. Traditionally, casting is a lengthy process, and several cast changes are frequently required to achieve the desired ROM. The standard practice has been to change casts every 5 to 7 days (61). Pohl et al (61) perform cast changes at much shorter intervals ranging from 1 to 4 days. Using this wearing schedule, they have observed equally effective ROM outcomes in shorter periods with fewer complications.
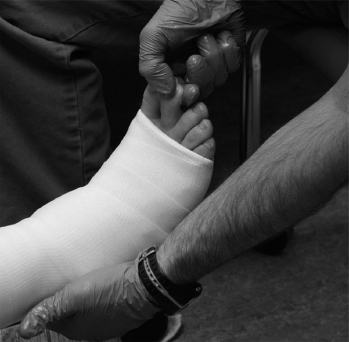
FIGURE 15.4 Clinician performing checking of capillary refill, a critical component of checking the limb after casting.
Possible complications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






