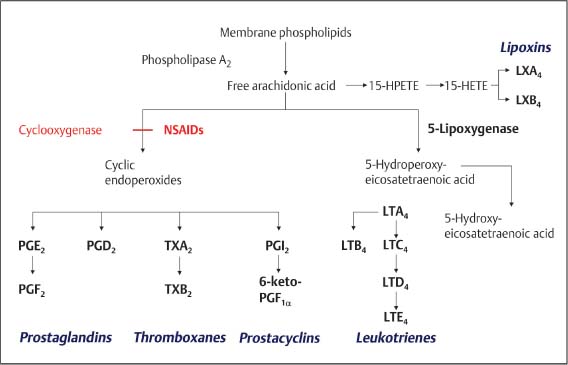
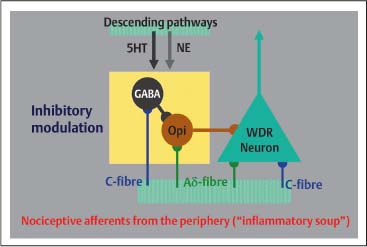
5 The Pharmacologic and Psychologic Treatment of Chronic Pain Several aspects should be considered in pharmacologic and psychologic treatments of pain that are related to the modulation of nociceptive impulses or signals. In addition, in the case of chronic pain, neither pharmacologic nor psychologic treatment, employed in isolation, leads to satisfactory results for patients. Based on this clinical experience the combination of pharmacologic and psychologic treatment of chronic pain is usually the best choice. For these reasons pharmacologic and psychologic aspects of pain treatment are discussed in the same chapter. Some knowledge of the basic mechanisms of pain is necessary to understand the possibilities of pharmacologic and psychologic treatment of pain. It is important to understand the molecular mechanisms involved in generating impulses or signals, referred to as nociception, and the processes of translation and modulation of these impulses in the central nervous system, in particular the complex processes of modulation that may be crucial to chronic pain development. A comprehensive summary of the complex processes involved in generating and experiencing pain is beyond the scope of this chapter. Several recent reviews concern the biological basis of pain, and the interested reader is encouraged to consult some of these (e. g., Besson, 1999; Julius and Basbaum, 2001; Scholz and Woolf, 2002. Only a rough summary of the mechanisms will be provided here. Three different mechanisms of pain need to be considered: nociceptive, inflammatory, and neuropathic pain (Scholz and Woolf, 2002). Nociceptive impulses or signals originate from the electrical activity (action potential) of the peripheral terminals of unmyelinated C-fibers and thinly myelinated Aδ-fibers. Although no identifiable anatomical structure in the periphery deserves the term receptor, these fibers are considered to contain receptors that are indeed ion channels sensitive to mechanical stimuli, hydrogen ions, cold, or heat. Under circumstances potentially dangerous for tissue, these factors may generate a nociceptive impulse or signal, which is an action potential perceived as pain. This pain is referred to as nociceptive pain. Inflammatory pain, in contrast, is the result of a more complex process following tissue damage in which chemical mediators emerge (e. g., histamine, serotonin, bradykinin, prostaglandins, ATP, H+, nerve growth factor, TNF-α, endothelins, interleukins). These chemical mediators, frequently referred to as “inflammatory soup” (Besson, 1999; Scholz and Woolf, 2002), act on free nerve terminals and generate an action potential (i. e., nociceptive impulse or signal). A complex reaction of small vessels from the surrounding tissue cells also occurs in the inflammatory tissue (e. g., mastocytes) to activate or to modify the stimulus response of nociceptor afferents. In summary, tissue damage, due to trauma or inflammation, initiates biological processes responsible for generating the nociceptive impulses or signals. Acute pain is therefore considered to result from noxious (i. e., potentially harmful) messages are derived from the activation of free unmyelinated or thinly myelinated terminals found in cutaneous, muscular, and joint tissues and in certain visceral structures (Besson, 1999). Finally, different mechanisms in the peripheral or central nervous system have to be considered for neuropathic pain (Fields et al., 1998). Sensitized nociceptors may induce changes in central processing, possibly leading to spinal cord hyperexcitability by which input from mechanoreceptors by the Aβ-fibers (i. e., touch) is perceived as pain. This phenomenon explains essential features of neuropathic pain such as hyperalgesia (intensity of pain being higher then expected) and allodynia (pain that is perceived due to an influence which is usually not perceived as pain, e. g., a slight blow on the skin). Reorganization in the dorsal horn, considered to result from C-fibers degeneration, seems to be responsible for allodynia and is provoked by the activity of Aβ-fibers. Furthermore, following nerve lesion in particular, the sympathetic system may interact with spinal afferent neurons, further sensitizing nociceptors (Fields et al., 1998; Baron, 2000). One of the central mechanisms in the generation of pain impulses that has been studied in great detail (Fig. 5.1), and is best understood, is the synthesis of prostaglandins. Because this process considerably affects pain treatment, some aspects are briefly mentioned here. Damage to the cell’s phospholipid membrane leads to a release of eicosanoids (e. g., free arachidonic acid), which are metabolized as shown in Figure 5.1. Prostaglandins are synthesized from phospholipids during this metabolism. As indicated in Figure 5.1, the enzyme cyclooxygenase (COX) plays a central role in this process. It is important to note that various compounds emerge in the process of metabolism of phospholipids. Some of these are important for organisms’ function (e. g., renal flow, function of the endothelium and gastric mucosae). Others, such as prostaglandins, are involved in inflammatory reactions. Fig. 5.1 Biosynthesis of eicosanoids. (Adapted from Brune and Hinz, 2001.) Prostaglandins play a major role in acute pain due to sensitization of receptors (free nerve terminals of C-fibers and Aδ-fibers) to other chemical mediators making up the “inflammatory soup” (Besson, 1999). In particular, because prostaglandins are involved in the sensitization and activation of free nerve terminals and prostaglandins are synthesized by the enzyme cyclooxygenase (COX), COX inhibitors have become a frequent choice in treatment of acute pain. Prostaglandins also help to establish structural changes in the synapses, thereby increasing transmission in the synapses of the central nervous system, particularly in the dorsal horn of the spinal cord. COX is therefore important for pain in the acute phase. However, according to recent research, many mechanisms remain elusive. In chronic pain, i. e., when there is no apparent tissue lesion or when an inflammatory reaction is not evident (for which rheumatoid arthritis may be an exception), COX may be of limited importance. Other grounds for chronic pain must accordingly be considered. In 1965 Melzack and Wall set a milestone in the understanding of the pain phenomenon with their gate-control theory (Melzack and Wall, 1965). This theory was the first to integrate nociceptive impulses as well as emotional and cognitive aspects of pain experience. Gate-control theory offered a framework that contributed to the comprehensive understanding of how chronic pain can develop. This theory works with anatomical structures and uses a so-called gate-system in the dorsal horn of the spinal cord, in which processing and modulation of the nociceptive impulse takes place. The following very simplified summary (see also Fig. 5.2) may be helpful in understand this process. As mentioned earlier, the nociceptive impulse reaches the dorsal horn of the spinal cord through unmyelinated C-fibers and thinly myelinated Aδ-fibers, where they are switched on to the second neuron (labeled “WDR neuron” for ‘wide dynamic range neuron’ in Figure 5.2 as it receives afferent input from different fibers). In addition, C- and Aδ-fibers give simultaneous, collateral impulses to GABA-ergic and opioidergic neurons of the spinal cord (Fig. 5.2). These GABA-ergic and opioidergic neurons reduce the excitatory influence on the WDR neuron by their action, and raise the stimulus threshold (i. e., the WDR neuron becomes inhibited). At the same time, descending pathways from the midbrain also act on WDR, GABA-ergic and opioidergic neurons via norepinephrinergic (NE) and serotoninergic (5HT) pathways. These descending pathways then additionally contribute to the inhibition of the WDR neuron. The nociceptive impulses are modified due to these processes, and pass up the spinal cord and through the thalamus to the cerebral cortex where they are perceived as pain. Once nociceptive impulses enter the central nervous system (i. e., “gate open”) this information is spread out to different parts including spinal cord, medulla, and cortex. Reflex phenomena are triggered (e. g., on the level of the spinal cord) due to spreading of the nociceptive impulses and endocrine reactions may follow (e. g., induced by the hypothalamus). In terms of triggering cognitive and emotional activity, the same impulses are finally processed in the brain cortex. Regarding descending modulation due to NE and 5HT, it is important to note that these neurotransmitters are critically involved in psychologic functioning, in particular the regulation of the emotional and affective status of a person. Conclusions of importance for a treatment strategy in chronic pain that can be drawn from the processes involved in the modulation of pain are: (a) Insufficient inhibitory modulation of the centripetal nociceptive impulses is central to the development of chronic pain; and (b) both physiologic and psychologic influences exert an inhibitory modulation on the nociceptive impulses by the appropriate pathways and are responsible for the development of chronic pain. The modulation of nociceptive impulses as outlined above is a restraining of the nociceptive impulse. Experimental investigations have shown that inhibitory modulation is insufficient with therapy-resistant and/or chronic pain (Besson, 1999). Research has also demonstrated that neurobiological changes of the WDR neuron may take place (Besson, 1999; Hoheisel et al., 1994) because of inadequate inhibitory modulation of the nociceptive impulse. These changes may occur in a very short time and can briefly be described as follows: The nociceptive impulses arise, for example, from an inflammation or a peripheral lesion in which the chemical mediators in the so-called “inflammatory soup” play a role. These mediators generate the nociceptive impulses (C- and Aδ-fibers), thereby stimulating the WDR neuron. The sustained nociceptive stimulation of the WDR neuron (mainly by the glutamatergic C-fibers) results in a sustained depolarization (i. e., excitation) of this neuron. In this case, the binding of glutamate in the C-fibers on the WDR neuron’s N -methyl-D-aspartate (NMDA) receptor is crucial. This receptor is responsible for controlling calcium ion channels. Opening calcium ion channels, among other actions, sets the second-messenger system in motion, followed by the transcription of the genetic information and increased gene expression (by the so-called immediate early genes). This expression is followed by increased reproduction of NMDA receptors on the WDR neuron. This appears to be a biologically significant mechanism that should protect the WDR neuron from excessive stimulation. However, changes in biological characteristics of the WDR neuron can result from an increased density of NMDA receptors on the neuron. This may have the following consequences: (1) since there is a higher density of NMDA receptors on the WDR neuron, many presynaptic impulses (which are often numerous) can contribute to the sustained depolarization of this neuron. In relation to chronic pain, the most important consequence is that due to changes in the WDR neuron mentioned above, nonnociceptive impulses may excite this neuron and be perceived as pain; and (2) as a result of biological changes, the WDR neuron shows an unusually high spontaneous discharge which, once again, is perceived as pain. These biological changes are also known as the “wind-up” phenomenon and represent the basis of central sensitization (Besson, 1999; Dickenson, 1995). Considerable importance is currently given to the process of central sensitization in the development of chronic pain. Given this central sensitization, and in particular the fact that the biological changes of the WDR neuron that have been described can take place rapidly (Hoheisel et al., 1994), an important aspect of pain treatment is the prevention or reversal of central sensitization. It is known that under some circumstances the WDR neuron may change its biological properties either to fire at a higher rate or to fire under the influence of many other afferent impulses. In this case even nonnociceptive impulses may lead to pain (such as simple limb movement, slight tissue pressure). This gives the impression of over-sensitivity when pain results from mild (i. e., usually non-pain-inducing) stimuli, and may be seen as a psychologic problem of the patient. Indeed this frequent clinical observation is based on hypersensitivity of the central nervous system. Once hypersensitivity is established, treating pain with pharmacologic agents may only increase problems. Fig. 5.2 Gate-control theory: scheme of modulation of the nociceptive impulses. Given that chronic pain results from changes in biological properties of the WDR neuron, the principal issue will be to act on modulation (i. e., downregulation of the impulse ratio). Careful consideration of the facts outlined above in treating chronic pain leads to the conclusion that prescription of prostaglandin synthesis inhibitors alone is of limited value. These chronic pain agents may have some influence on nociceptive impulses (e. g., in rheumatoid arthritis where continuous tissue damage may take place). COX inhibitors may have an additional positive effect in reducing the rate of synaptic transmission in the central nervous system, which is under the influence of prostaglandins. It is more important to consider that treatment of chronic pain using only pharmacologic agents may fail because the doses required for complete analgesia may be greatly exceed the level at which toxicity occurs. The treatment benefit for chronic pain while using NSAIDs is only very limited because influence on downregulation of the WDR neuron cannot be provided. This indicates that in chronic pain the pharmacologic treatment will have to focus on different pharmaceutical substances, and that other nonpharmacologic strategies may be helpful. Given that in many chronic pain syndromes the downregulation of the WDR neuron is required, NSAIDs may be employed rather than opioids. In acute pain, particularly postoperative pain or other severe pain, or moderate to severe chronic pain opioids appear to be the drug of choice. This is clearly supported by extensive clinical experience. The choice of opioid, route of administration, and sometimes variation between the drugs should be carefully considered. Opioids (from opium, which is the Greek term for juice, i. e., extract from the poppy plant) are a group of morphinelike substances with primarily analgesic properties. The pharmacologic effects of all opioids are based on their interactions with the three opioid receptors mu (μ), kappa (κ), and delta (δ), which were discovered in the early 1970s. The mu receptor is the principal structure in the analgesic action of opioids. Opioid receptors exist in the periphery, in the spinal dorsal horn, in the brainstem, in the thalamus, and in the cortex. The main effects of opioids include a decrease of presynaptic transmitter release, hyperpolarization of postsynaptic neurons, and disinhibition. The discovery of opioid receptors encouraged the search for endogenous substances that might be responsible for modulation of action. This research identified enkephalins, endorphins, and dynorphins. In the 1980s precursor molecules of endogenous opioid-receptor agonists were identified: preenkephalin, proadrenocorticotropic hormone (pro-ACTH), ACTH endorphin (propiomelanocortin), and prodynorphin. The localization of the endogenous opioids was identified. Enkephalin is found in the amygdala, hypothalamus, the midbrain periaqueductal gray matter, the rostroventral medula and the dorsal horn of the spinal cord. β-Endorphin is mainly found in the hypothalamic arcuate nucleus and the midbrain periaqueductal gray matter. Dynorphins have similar distribution to enkephalins (Fields, 1987).
Understanding Pain Mechanisms
Pain Mechanisms
Gate-Control Theory
Conclusions
Pharmacologic Treatment of Chronic Pain
Opioids
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree