Chapter 4.4 The influence of pH and other metabolic factors on fascial properties (H+: hydrogen ion, Furthermore, the Henderson–Hasselbach equation gives an idea how this buffer system works: Figure 4.4.1 gives an idea how the most important organs, the kidney and lungs influence the bicarbonate–acid buffer. Physical exercise, for example, leads to a significant increase of acidic metabolites such as lactate and CO2 due to glycolysis and the cellular respiratory chain. An increase of the partial CO2 pressure in the blood, measured in the brain stem and in peripheral chemoreceptors located in the aortic arch and carotid arteries, is the most dominant breathing stimulator for the breathing center located in the brain stem. Activation of the breathing center leads to deeper and faster breathing, thereby increasing ventilation. Thereby CO2 can be eliminated, which helps to keep the blood pH constant. Furthermore, under pathophysiological conditions – like in septic patients with a metabolic acidosis (pH < 7.3, and low buffer base) – breathing is often enormously activated to counteract the metabolic acidosis. Instead, patients with a metabolic alkalosis (pH > 7.5, and high buffer base), for example due to excessive vomiting, may show a depression of respiration with an increase of partial CO2 pressure in the blood to keep the pH in the physiological range. However, this compensation mechanism is limited, because hypoventilation is only tolerated in narrow ranges. The kidneys provide a mechanism of saving basic metabolites by regeneration of the bicarbonate ( Chronic hyperventilation – also called “breathing pattern disorder” – tends to lead to a state of reduced CO2 in the blood, known as hypocapnia. Hypocapnia goes along with increased alkalosis and leads to vasoconstriction as well as to increased nerve and muscle excitability. Hypocapnic alkalosis has been observed in anxiety as well as in other negative affective states and traits (Chaitow et al. 2002). Reversing sustained or spontaneous hyperventilation with therapeutic capnometry has proven beneficial effects in the treatment of panic disorder as well as asthma (Meuret & Ritz 2010). Interestingly, patients with psychogenic hyperventilation frequently show elevated lactate levels. While high lactates are usually associated with acidosis, it has recently been shown that in patients with psychogenic hyperventilation this correlation is not valid, due to adaptation processes (Ter Avest et al. 2011). In this connection, it is very interesting that psychiatric disorders like panic disorders (PD) with chronic or acute hyperventilation may have an influence on fascial function. For example, it has been shown that patients with PD have a significantly, higher incidence of joint hypermobility syndrome (Martin-Santos et al. 1998). In another study, patients with PD suffered significantly more often with prolapse of the mitral valve, also indicating more lax connective tissue (Tamam et al. 2000). However, there are other studies that could not detect any significant relationship between PD and joint hypermobility syndrome or mitral valve prolapse (Gulpek et al. 2004). Furthermore, the exact pathomechanism behind these findings is still unknown. Genetic studies, for example, have looked at elastin polymorphism and could not find any association with PD (Philibert et al. 2003). It is known that patients with PD often have electrolyte disturbances, like hypophosphatemia (indicator of chronic hyperventilation), elevated lactate levels, and increased CO2 sensitivity (Sikter et al. 2007). Whether these electrolyte disturbances may be explanations for the higher incidence of lax connective tissue in patients with PD has to be proven in the future. Acid–base status is strongly linked with the electrolyte balance in the cells. Protons (H+) and potassium (K+) are the cations to which resting cellular membranes are permeable. This is the reason why H+ and K+ supersede each other in order to hold an electrochemical equilibrium across the membranes. However, the effect of acid–base status on the serum potassium is very complicated and depends on the nature of the disorder. In general, extracellular K+ concentration rises in acidic conditions and falls in alkalosis. This is important because K+ is stabilizing the resting membrane potential and K+ deviations can lead to heart arrhythmia, for example, and muscle fatigue. However, exercise can lead to a significant increase of extracellular K+ in the muscle. Indeed, K+ levels rise significantly after extensive physical exertion. Emergency medics need to treat cardiac arrhythmias in almost every marathon event, where of course factors other than high K+ contribute to heart instability. The K+ accumulation in the muscular microenvironment (i.e., within the transverse tubular system) has also been linked to muscle fatigue, which is counteracted by simultaneous elevation of muscle temperature, lactic acidosis, and the presence of endogenous catecholamine (Pedersen et al. 2003).
pH regulation and influence on fascial tissue
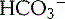 : bicarbonate ion, H2CO3: carbonic acid, H2O: water, CO2: carbon dioxide.)
: bicarbonate ion, H2CO3: carbonic acid, H2O: water, CO2: carbon dioxide.)
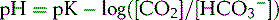
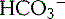 ) and secretion of H+. Moreover, the kidney is important in respiratory diseases, like chronic obstructive pulmonary disease, where the patients suffer from a chronic high partial CO2 in the blood, which induces a so-called respiratory acidosis (high CO2, normal bicarbonate at the beginning). The kidney has the ability to counteract this acidosis by regeneration of more bicarbonate and an increase of acid secretion with the urine. In contrast to the lung, the counteraction of the kidney to keep the pH constant is slower.
) and secretion of H+. Moreover, the kidney is important in respiratory diseases, like chronic obstructive pulmonary disease, where the patients suffer from a chronic high partial CO2 in the blood, which induces a so-called respiratory acidosis (high CO2, normal bicarbonate at the beginning). The kidney has the ability to counteract this acidosis by regeneration of more bicarbonate and an increase of acid secretion with the urine. In contrast to the lung, the counteraction of the kidney to keep the pH constant is slower.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


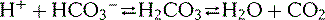
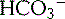 in the blood. This ratio is relatively constant, because the concentrations of both buffer components are very large, compared with the amount of H+ added or removed in the body during normal circumstances. Under heavy exercise or pathophysiological situation, the added H+ protons may be too great for the buffer alone to control the blood pH. When this happens, another organ must help to maintain a constant pH in the blood.
in the blood. This ratio is relatively constant, because the concentrations of both buffer components are very large, compared with the amount of H+ added or removed in the body during normal circumstances. Under heavy exercise or pathophysiological situation, the added H+ protons may be too great for the buffer alone to control the blood pH. When this happens, another organ must help to maintain a constant pH in the blood.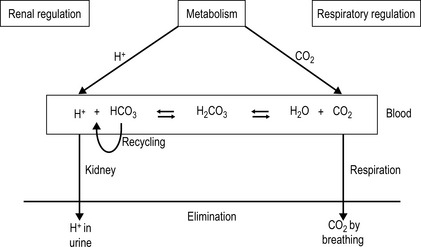
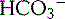 ). Metabolic alkalosis is counteracted by hypoventilation and thereby decreased CO2 elimination. Respiratory acidosis is reversed by an increased H+ secretion and reabsorption of (
). Metabolic alkalosis is counteracted by hypoventilation and thereby decreased CO2 elimination. Respiratory acidosis is reversed by an increased H+ secretion and reabsorption of (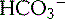 ) and vice versa in respiratory alkalosis.
) and vice versa in respiratory alkalosis.





