Major featuresb
Located anywhere along the femur from just distal to the lesser trochanter to just proximal to the supracondylar flare
Associated with no trauma or minimal trauma, as in a fall from a standing height or less
Transverse or short oblique configuration
Noncomminuted
Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex
Minor features
Localized periosteal reaction of the lateral cortexc
Generalized increase in cortical thickness of the diaphysis
Prodromal symptoms such as dull or aching pain in the groin or thigh
Bilateral fractures and symptoms
Delayed healing
Comorbid conditions (e.g., vitamin D deficiency, RA, hypophosphatasia)
Use of pharmaceutical agents (e.g., BPs, GCs, PPIs)
Based on these previous results, the task force summarized that AFFs are rare, particularly when considered in the context of the millions of patients who have taken BPs and also when compared with typical and common femoral neck and intertrochanteric fractures (Fig. 6.1). The task force also emphasized that while BPs are important drugs for the prevention of common osteoporotic fractures, the occurrence of AFFs is a matter of concern, and that there is an urgent need for to assist in identifying the patients who are at particular risk of developing AFFs and to guide decision-making regarding the duration of BP therapy. Physicians and patients should be made aware of the possibility of AFFs and of the potential for bilaterality. Given the relative rarity of AFFs (Fig. 6.1) [17], the task force emphasized the facilitation of future research, especially with regard to case reporting based on the case definition that is established.
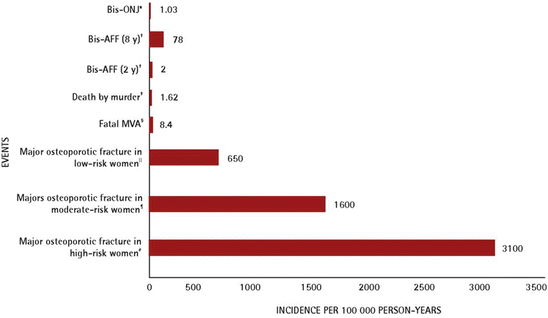

Fig. 6.1
Risks of major osteoporotic fracture and other rare events. Bis-AFF bisphosphonate-associated atypical subtrochanteric and diaphyseal femur fracture, Bis-ONJ bisphosphonate-associated osteonecrosis of the jaw, BMD bone mineral density, FN femoral neck, FRAX fracture risk assessment tool, MVA motor vehicle accident. *[16] (Reprinted with permission from the college of family physicians of Canada [17]) (†: Data from Dell R, Greene D, Ott SM, Silverman S, Eisemon E, Funahashi T, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009 [abstract]. J Bone Miner Res 2010:25; ǂ: Data from Statistics Canada, Homicide offences, number and rate, by province and territory. Ottawa: Statistics Canada; 2011; §: Data from Transport Canada. 2007 casualty rates. Ottawa: Transport Canada 2010; ‖: The 10-year risk of major osteoporotic fracture in a low-risk woman by Canadian FRAX (65-year-old woman, weighing 60 kg with a height of 168 cm; BMD FN T-score –1.2); ¶: The 10-year risk of major osteoporotic fracture in a moderate-risk woman by Canadian FRAX (65-year-old woman weighing 60 kg with a height of 168 cm; parent hip fracture history; BMD FN T-score -2.0); #: The 10-year risk of major osteoporotic fracture in a high-risk woman by Canadian FRAX (65-year-old woman weighing 60 kg with a height of 168 cm; parent hip fracture history; previous fracture; BMD FN T-score -2.6))
6.2.2 The ASBMR Task Force Report, 2nd Edition
Following the publication of the first edition of the ASBMR task force report in 2010, several studies were published on the epidemiology of AFFs, their risk factors, and their relationship with the use of BPs. The ASBMR reconvened the task force at the 2012 Annual Meeting of the ASBMR. The first goal of the task force was to review the major studies that had been published since the original task force report in 2010, with a focus on studies that addressed three major aspects of AFFS: their epidemiology , pathogenesis , and medical management . The second goal was to assess whether the information in the reviewed studies provided data that could be used to refine the original case definition. Following these processes, the task force published the 2nd edition of the ASBMR task force report on the AFFs (Table 6.2) [18].
To satisfy the case definition of AFF, the fracture must be located along the femoral diaphysis from just distal to the lesser trochanter to just proximal to the supracondylar flare |
In addition, at least four of five major features must be present. None of the minor features are required but have sometimes been associated with these fractures |
Major featuresa |
The fracture is associated with minimal or no trauma, as in a fall from a standing height or less |
The fracture line originates at the lateral cortex and is substantially transverse in its orientation, although it may become oblique as it progresses medially across the femur |
Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex |
The fracture is noncomminuted or minimally comminuted |
Localized periosteal or endosteal thickening of the lateral cortex is present at the fracture site (“beaking” or “flaring”) |
Minor features |
Generalized increase in cortical thickness of the femoral diaphyses |
Unilateral or bilateral prodromal symptoms such as dull or aching pain in the groin or thigh |
Bilateral incomplete or complete femoral diaphysis fractures |
Delayed fracture healing |
The reports were revised to more clearly delineate the features that distinguish AFFs from ordinary osteoporotic femoral fractures. New epidemiologic studies, many of which incorporated radiographic reviews and provided new information on the incidence of AFFs and the association with BPs, and new data on the pathogenesis and management of AFFs were reviewed and summarized in the second report. The features linking AFFs to comorbid conditions and medication exposures, including BPs and GCs, were removed, because it was deemed more appropriate for studies to seek these associations than to include them in the case definition.
6.3 Epidemiology
There are two types of published epidemiologic studies on AFFs: large registry-based cohort studies to demonstrate the incidence of subtrochanteric fractures (STs) and femoral shaft fractures (FSs) and studies in which AFFs were categorized based on radiographic findings. In the first type of studies, the overall incidence of STs and FSs was evaluated in registry-based cohort studies using a large database, such as the International Classification of Diseases (ICD) codes [19–22]. The studies lacked radiographic adjudication in differentiating the STs and FSs from the typical osteoporotic femur fractures from AFFs. The studies demonstrated that the age-adjusted rates for hip fractures decreased by 31.6 % from 1996 to 2006, while the number of STs and FSs increased by 31.2 % [23]. The rate of STs and FSs among overall hip fractures increased, and the incidence of STs and FSs in females was between 10 and 35 per 100,000 person-years. Although this trend indirectly suggests a relationship between the increased use of BPs and the incidence of AFFs, the actual risk could not be estimated from these types of studies. Therefore, while the large database-based studies are helpful for estimating the incidence of AFFs, it is hard to determine the actual number of AFFs from these studies.
In the second type of studies, AFFs were determined and categorized based on the reviewing of radiographs [11, 24–33]. Thirty-two percent of the overall STs and FSs treated at the Hospital for Special Surgery in New York from 2000 to 2007 were AFFs [29]. In Australia, 13 % of 152 patients with STs and FSs were AFFs [27]. In the Netherlands, 16 % (10/63) of STs and FSs were AFF [28]. In the reviewing of 1234 radiographs of STs and FSs that occurred in Sweden in 2008, approximately 5 % were found to be AFFs [32]. Among 3515 patients with femoral fractures in the United Kingdom, 27 AFFs were identified, which represented 0.8 % of all hip fractures and 7 % of FSs [33]. In Japan, we identified 14 AFF cases among 402 STs and FSs (3.5 %) by reviewing the radiographs of 2238 hip and FS fractures [10].
Based on these radiographic adjudication studies and large database studies, the incidence of AFFs is estimated to be between 0.3 and 11 per 100,000 person-years. Similarly, in the United States, the incidence of AFFs among the femur fractures in females over 50 years of age and males over of 65 years of age between 1996 and 2009 was 5.9 per 100,000 person-years [34]. In Switzerland, the incidence rate of AFFs was 3.2 per 100,000 person-years [31].
6.4 Risk Factors
Several case–control studies with radiographic adjudication have compared typical osteoporotic fractures with AFFs [10, 27, 31, 32, 34, 35]. In Australia, 152 femoral fractures (not including hip fractures), which occurred in 152 patients (mean age 78 years) from June 2003 to May 2008, were reviewed [27]. Twenty of the 152 fractures (13 %) were classified as AFFs, and 17 of the 20 AFF patients (85 %) were BP users, while three of 132 patients with typical femoral fractures (2.3 %) were taking BPs. The relative risk of an AFF patient being on a BP was 37.4 (95 % CI, 12.9–113.3; p < 0.001). AFFs were also associated with other factors, such as a history of a low-energy fracture (odds ratio (OR), 3.2; 95 % CI, 2.1–17.1; p <0.001), the use of glucocorticoid (GCs) for more than 6 months (OR, 5.2; 95 % CI, 1.3–31.0; p = 0.01), active rheumatoid arthritis (OR, 16.5; 95 % CI, 1.4–142.3; p < 0.001), and a serum level of 25-hydroxyvitamin D of less than 16 ng/ml (40 nmol/l) (OR, 3.5; 95 % CI, 1.7–18.7; p < 0.001).
In Sweden, when 1234 radiographs of 1271 female patients with STs and FSs were reviewed, 59 patients showed AFFs [32]. As 78 % of the AFF patients and 10 % of the controls had received BPs, the multivariable-adjusted OR was 33.3 (95 % CI, 14.3–77.8). The duration of BP use influenced the risk (OR per 100 daily doses, 1.3; 95 % CI, 1.1–1.6). After drug withdrawal, the risk diminished by 70 % per year after the last use (OR, 0.28; 95 % CI, 0.21–0.38).
In the United States, when the 22 typical FS fractures and the 75 AFFs were reviewed, the adjusted OR of ever taking OR in patients with AFFs vs. non-atypical FSs was 2.11 (95 % CI, 0.99–4.49) [34]. The ORs for exposure to GCs and PPIs did not differ significantly between the groups.
In Switzerland, the fracture of 39 of 477 patients with STs and FSs, who were older than 50 years of age between 1999 and 2010, and who were hospitalized at a single university medical center, was identified as AFFs [31]. Eighty-two percent of the patients with AFFs had been treated with BPs, while 6 % of the patients with typical femoral fractures had been treated with BPs (OR, 66.9; 95 % CI, 27.1–165.1). While the OR for AFFs in patients who had used BPs for less than 2 years was 35.1 (95 % CI, 10.0–123.6), the ORs for AFFs in patients who had used BPs for 2–5 years, 2–9 years, and more than 9 years were 46.9 (14.2–154.4), 117.1 (34.2–401.7), and 175.7 (30.0–1027.6), respectively.
In New Zealand, there were six AFFs among 65 typical femoral fractures (TFFs) [35]. Half of the patients (50 %) with AFFs used BPs at the time of the fracture, while 23 % of the patients with TFFs used BPs (OR, 5.5; 95 % CI, 0.97–31; p = 0.07). In addition, the OR for exposure to GCs was 4.9 (95 % CI, 0.74–32.7; p = 0.13).
In Japan, we conducted a case–control study in which ten patients with AFFs were compared with 30 patients with low-energy typical STs and FSs [10]. In the AFFs group, 90 % of the patients used BPs, whereas 14.3 % of the patients in the TFFs group used BPs. A fracture location-, age-, and gender-matched (1:3) case–control study revealed that the administration of BPs and GCs and the presence of a collagen disease (CD) were risk factors for the development of AFFs (OR: 36.0 [95 % CI, 3.8–342.2], 13.0 [2.3–74.1], and 9.0 [1.6–50.3], respectively).
In a prospective study of the Healthy Bones Program in Kaiser, Southern California, 142 patients with AFFs were found among all of the femur fractures form 2007 to 2011, in 1,835,116 patients who were older than 45 years of age [26]. One hundred twenty-eight of 142 patients with AFFs (90 %) were BP users. The occurrence of AFFs was not correlated with the duration of use (5.5 years), age (69.3 years of age), or bone density (T-score, −2.1). As 188,814 patients used BPs, the age-adjusted incidence rates for AFF were 1.78/per 100,000/person-years (95 % CI, 1.5–2.0) with 0.1–1.9 years of exposure and increased to 113.1/per 100,000/person-years (95 % CI, 69.3–156.8) with 8–9.9 years of exposure. The incidence of AFFs increased with a longer duration of BP use .
According to these studies, the incidence of AFFs is higher in patients taking BPs, and a longer duration of BP use was related to an elevated risk of developing AFFs. Consistent with these results, the systematic review and meta-analysis examining the association of BPs with AFFs showed that the adjusted relative risk (RR), based on case–control studies with radiographic adjudication, was 11.12 (95 % CI, 2.68–46.18) [36]. These findings indicate that BP use seems to be a risk factor for AFFs and that the risk seems to increase in line with the duration of BP use.
The adherence to oral BPs of 522,287 new BP users was followed among all Medicare fee-for-service female beneficiaries in the United States from 2006 to 2010 [37]. The age-adjusted incidence rates for intertrochanteric fractures (ITs) and femoral neck fractures (FNs) among the highly compliant beneficiaries were significantly lower than those among less compliant users (adjusted hazard ratio [HR] = 0.69; 95 % CI, 0.66–0.73). On the other hand, HR of STs and FSs for the highly compliant users vs. the less compliant users became significant after 2 years of follow-up (HR = 1.51; 95 % CI, 1.06–2.15) and reached the highest risk level in the fifth year (HR = 4.06; 95 % CI, 1.47–11.19).
6.5 Pathogenesis
The mechanisms underlying the development of AFFs have not been fully understood. The characteristics of the radiological features of AFFs, such as focal hypertrophy of the lateral cortex, periosteal and endosteal callus formation, and the transverse fracture line at the lateral cortex, suggest that fatigue damage accumulates within the bone cortex over long period of time and that AFFs are stress or insufficiency fractures. A concentration of mechanical stress on the bone leads to the formation of microcracks , which heal by bone remodeling via the initiation of osteoclastic bone resorption , followed by osteoblastic bone formation to replace new bone. The pathogenesis of BP-associated fractures seems to be related to the alterations of this tissue repair process as a result of the continuous suppression of the bone turnover rate [39].
The reduction of process of the bone turnover by BP treatment alters the bone mineral and matrix properties. BP therapy causes an increase in advanced glycation end products of the extracellular bone matrix, deteriorating the mechanical properties of the bone [5]. Prolonged BP therapy causes the accumulation of microdamage to bone and reduces the heterogeneity of the organic matrix and mineral properties [4]. These changes reduce the bone repair ability, which results in the accumulation of local microdamage especially at the site of maximum mechanical force.
Several reports have investigated the histology of the AFF patients. The ASBMR task force summarized them in 2010 [6]. Most of the histological analyses involved the performance of transiliac bone biopsies on samples from the patients with BP-associated AFFs, which revealed the reduced or absent populations of osteoclasts and osteoblasts , suggesting that a relationship existed between these phenomena and BPs. In contrast to the transiliac bone biopsies, a small number of femoral bone biopsies reports have been conducted. The histomorphometric analysis of a biopsy from the femur of an AFF patient revealed a normal lamellar bone texture, no evidence of adynamic bone, and no impairment of the mineralization, suggesting that there was no association between the AFFs and the over-suppression of bone turnover caused by BP use [40]. However, another study reported (based on a histological analysis of femur biopsies from eight patients with AFFs) that AFFs appeared to consist of a microcrack at the lateral cortex with its main direction perpendicular to the long axis of the bone [41]. The surrounding bone showed the signs of remodeling, mainly represented by the presence of osteoclasts, resorption cavities, and woven bone facing the crack. However, half (4/8) of the AFFs consisted mainly of empty lacunae in osteocyte lacunae. These results suggested that the impaired healing of the cracks, in spite of increased remodeling of the adjacent bone, was related to the development of AFFs in BP users.
Based on these studies, the pathophysiology of AFFs is suggested to be related to the impairment of the bone repair process , where microcracks need to be remodeled to new bone, due to a long-term decrease in bone turnover caused by the suppression of the osteoclast function by antiresorptive agents, such as BPs and, presumably, denosumab (Fig. 6.2) [42].
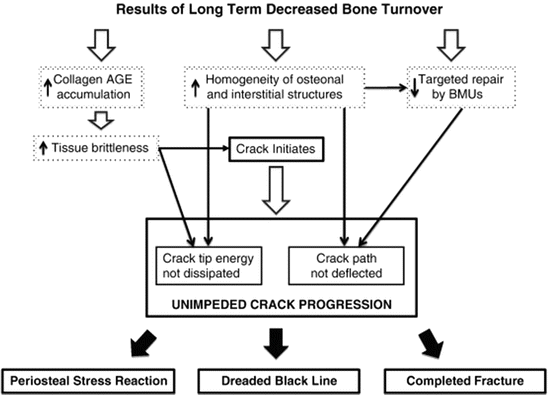

Fig. 6.2
Flow diagram of pathophysiological steps leading from suppression of bone turnover to atypical femoral fracture. AGEs advanced glycation end products, BP bisphosphonate exposure, BMU bone metabolic unit, AFF atypical femoral fracture (Reprinted with permission from the Elsevier [42])
6.6 Femoral Geometry and the Biomechanical Considerations of the Lower Limb
A “stress fracture ” implies the abnormal and excessive loading of a normal bone, while an “insufficiency fracture ” implies the normal loading of an abnormal or deficient bone. Bones subjected to repetitive loading that overwhelms the body’s capacity for repair are at risk of developing a stress fracture. Thus, stress fractures which result from repetitive mechanical loading on the bone are common overuse injuries in physically active individuals, such as athletes. A stress fracture results from repetitive application of stress of lower strength than that required to fracture bone in a single load. While stress fractures can affect almost any bone, the vast majority of these fractures occur in the lower extremities. As the vague symptoms may lead to delayed diagnosis and an increased risk of major complications, the early detection of high-risk stress fractures, such as the tension side of the femoral neck, the tarsal navicular, and the base of the fifth metatarsal, is important [43]. Stress fractures, which most commonly occur in the tibia, constitute about 10 % of all sport-related injuries [44]. Stress fractures of the femur are relatively uncommon, and the data from the literature suggest that they constitute less than 5–10 % of all sport-related stress fractures [45–47]. Stress fractures of the femoral shaft are especially rare but they do occur in the proximal third of the femur [48–50]. In addition, most of the cases of the stress fractures of the femoral shaft occur in the medial side of the femora [51–53].
Stress is produced in the femur whenever it is subjected to a loading force. The femur is subjected to forces produced by the body’s weight several times during normal physiological activity. The femur is exposed both under tension and compression. The femur may be subjected to weight-bearing force below the lesser trochanter in the medial cortex. The femur is usually subjected to a lower force below the lesser trochanter in the lateral cortex (immediately opposite the medial cortex). Bending forces , rather than compressive or torsional forces, are considered to be important in the pathogenesis of the majority of stress fractures [54]. With regard to bending forces, two critical factors exist: (1) the geometric distribution of bone mass, rather than bone mass itself, and (2) the collagen composition of the bone. The bone mass is controlled, at least in part, by bone remodeling. The most widely accepted theory about the development of stress fractures is based on an imbalance in this process. The frequency of the magnitude of load and the number of repetitions are factors that define the fatigue process. Repetitive stress causes periosteal resorption that occurs at a faster rate than bone formation. The cortex, as a result, is weakened and becomes fractured, a process called microdamage . This microdamage within the bone develops with each loading cycle. The bone is unable to adapt to this stress in moderate and high doses of exercise. As a result, cumulative microdamage results in cracks in the bone that act as stress raisers and which allows for the development of fractures. These fractures may be incomplete, complete, nondisplaced, or displaced. Hormonal disorders and nutritional deficiency may impair the normal response of the bone to stress. Certain characteristics of the lower extremities also may influence the stress response of bone by altering the transfer of load to the femur, such as inequality in leg length, coxa vara, and cavus feet [55]. Overall, stress injury to the bone represents a spectrum of diseases, from bone stress reactions to stress fractures, which may initially be subclinical.
A case report demonstrates the initial development of a periosteal callus and the eventual appearance of a transverse cortical fracture in the region of periosteal thickening termed the “dreaded black line ” [56]. This pattern is typical of the development of a stress fracture. Based on the evidence of periosteal and endosteal callus, and on the appearance of a transverse cortical fracture prior to the overt fracture, the current consensus of the task force is that AFFs are stress or insufficiency fractures that develop over time. However, AFFs differ from exercise-induced femoral stress fractures in some respects.
The initiation of exercise-induced femoral stress fractures usually occurs on the medial cortex of the femur, in the proximal one-third of the femoral diaphysis, which results in a more oblique fracture surface than is observed in AFFs [57]. In contrast, the initiation of AFFs occurs on the lateral cortex, between the lesser trochanter and the femoral condyles, and results in a smooth transverse surface, which is more characteristic of a brittle material. The lateral cortex of the femur is known to sustain high levels of tensile stress due to bending [58, 59]. Based on these data, it has been suggested that the tensile stress of the lateral cortex of the femur may precipitate the damage in this location, especially in people with lower limb geometry that could exacerbate that effect, such as bowed femur and Asian race .
The geometry of the hip and proximal femur determines, in part, the stresses that are experienced on the lateral aspect of the femoral cortex. In addition, AFFs occur not only in BP users but also in BP-naïve individuals. Thus, the pathophysiology of AFFs cannot be entirely explained by BP usage. The increased tensile stress on the lateral cortex would explain the subtrochanteric and diaphyseal distribution of AFFs.
While AFF can occur anywhere in the femoral bone from just beneath the lesser trochanter to the femoral shaft [6, 18], it remains unclear how the fracture site of an AFF is determined [28, 31, 34, 60]. The patterns in the radiological findings of AFFs, such as cortical thickening , beaking , and flaring of the lateral cortex of the femoral shafts, suggest that this type of fracture is caused by tensile failures of the lateral cortex of the femoral shaft [6, 15]. In addition to these radiological features, bilateral fractures are one of the important clinical characteristics of patients with AFFs [6, 28, 61]. In most cases of bilateral AFFs, the fractures occur in the same anatomical location [28]. This suggests that in the AFFs, individual factor(s), in addition to the other abovementioned factors, may affect the fracture sites. These clinical features suggest that AFFs, as least in part, develop due to the fatigue failure mechanisms that are observed in a stress fracture and which are related to bone tissue properties that become altered as a result of microdamage accumulation as well as altered adaptations to mechanical forces, which may be related to BP, and probably denosumab, treatment, and/or other conditions that can cause bone fragility.
Although long-term antiresorptive therapy may affect bone repair and its mechanical properties and also increase the susceptibility of the bone to stress fracture, the systemic bone changes caused by antiresorptive drugs cannot explain both the specific location where the onset of an AFF takes place and the recurrence of AFFs in a specific femoral region, namely, the lateral cortex of the femoral shaft. A possible explanation for AFFs may reside in the daily mechanical environment of the femoral shaft. Aamodt et al. directly measured the tensile strains in a single location of the lateral femoral shaft in two patients [62]. Theoretical investigations, using a synthetic femur to mimic walking, reported that femoral strain patterns were characterized by combined bending and torsion [63]. Martelli et al. hypothesized that the typical strain patterns in the femoral shaft were activity type dependent and that some of them created a more favorable condition for AFFs in the lateral cortex. The authors focused on the typical strain patterns in the femoral shaft during activities of daily living and reported the three-dimensional in vivo tensile and compressive strain distributions in the femoral shaft during different daily activities [59]. In this study, the cortical strain in the femoral shaft was studied by combining experimental motion data and models of femoral elasticity and forces. The lateral aspect of the femur was subjected to tensile strains during all of the investigated activities. There was an association between typical locations for AFFs and tensile strains in the femoral shaft while walking (Fig. 6.3). Both strain intensity and orientation in the transverse plane were activity dependent, and walking showed the highest average tensile strains in correspondence with the location of onset of commonly observed AFFs. The lateral femoral shaft was subjected to peak tensile loads during most of the walking stance phase, whereas the peak tensile strain was slightly rotated anteriorly during stair descent. The results of this study suggest that the location of the onset of AFF is associated with physiological strain distribution during daily activities and that the tensile loads in the lateral femoral shaft are to be attributed to the hip abduction moment. The lateral femoral shaft is subjected to tensile strains during a variety of physical activities and walking induces the highest tensile strain levels (Fig. 6.4). The hip abduction moment is a strong predictor of the tensile strains in the lateral femoral shaft during walking and stair ascent, but not during general activities. The lateral aspect of the femoral shaft was loaded in tension during every studied activity. The result of the multi-parametric linear regression analysis means that 46–60 % of the tensile strain variance in the lateral femoral shaft can be explained by the whole body dynamics, whereas the remaining 40–54 % of the strain variance is likely to be attributed to other unexplained factors. Therefore, the combination of tensile loads, in addition to the increased bone fragility that may be associated with long-term antiresorptive therapy, may be an important cofactors in creating a favorable environment for AFFs.
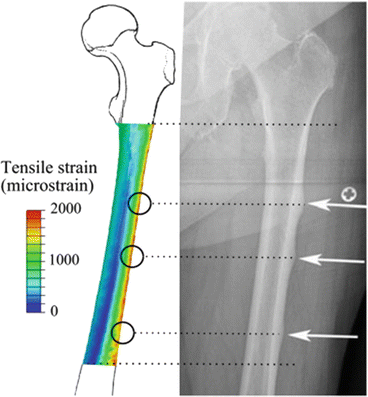
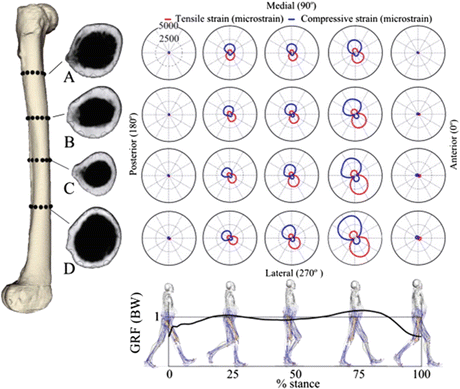


Fig. 6.4
Cortical tensile and compressive strain patterns in four transversal section of the femoral shaft at five time intervals during the stance phase of walking (Reprinted with permission from the Elsevier [59])
Nine consecutive elderly patients who were treated for low-energy diaphyseal femoral fractures between 2005 and 2010 at Akita University in Japan were retrospectively reviewed and the femoral curvature was suggested to be associated with the occurrence of AFFs [8]. Low energy was defined as a fall from standing height or less. As three of nine patients showed the opposite side of low-energy diaphyseal femoral fractures within a few years, a total of 12 femurs in nine patients were investigated in this report. All patients were female with a mean age at the time of first injury of 75.6 years (range 71–86 years). All nine patients used the medication for the treatment of osteoporosis at the time of fracture. BPs were used by eight of the patients, while the remaining patient used raloxifene. The mean duration of drug administration was 3.6 years. The curvature of the femur was measured with anteroposterior (AP) and lateral views as the angles between two linear lines drawn along the proximal and distal portion of the femoral shaft. The angle on the AP view was identified as angle A, while that on the lateral view was identified as angle B (Fig. 6.5). The femoral curvatures , defined by angles A and B, were significantly larger in the low-energy fracture group than in the control group (Table 6.3), suggesting that increased femoral curvature might be an important causative factor for low-energy diaphyseal femoral fractures.
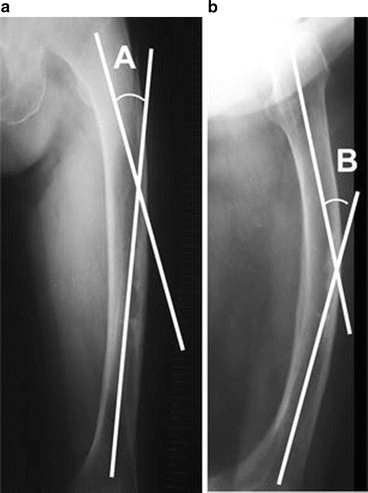

Fig. 6.5
Involvement of the femoral curvature for the atypical femoral fractures (AFFs). For the measurement of femoral curvature, two lines along the proximal and distal portions of the femoral shaft were drawn on anteroposterior (a) and lateral (b) X-rays. Angle A is defined as the angle between the two lines on the anteroposterior view and angle B as that on the lateral view (Reprinted with permission from the Springer [8])
Table 6.3
Comparisons of femoral curvature between the low-energy diaphyseal femoral fracture group and the control group [8]
(°) | AFFs (n = 7) | Control (n = 24) | p |
|---|---|---|---|
Angle A | 12.6 | 4.6 | 0.002 |
Angle B | 19.9 | 11.8 | 0.001 |
Thirteen cases of stress fractures of the bowed femoral shaft (SBF) were reviewed retrospectively among elderly Japanese patients [9]. All of the patients were females with a median age at injury of 77.0 years (range 67–88 years), who were able to walk independently before the injury and who met the AFF diagnostic criteria. Six of the 13 cases of AFFs showed a bowing deformity of the femur and used BPs, while one of the seven cases in which the bowing deformity was absent used BPs. The remaining six cases did not use BPs, showing that AFFs can occur in patients without using BPs. Based on the results of this study, the authors demonstrated the concept of the AFF criteria, as shown in Fig. 6.6. However, further study is required to fully reveal the pathophysiology of AFFs (e.g., whether there are cases of SBF who do not meet the radiographic definition of AFFs as defined by ASBMR task force) (Table 6.2) [18].
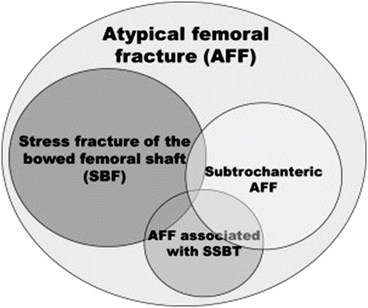

Fig. 6.6
New concept of atypical femoral fracture (AFF) in terms of mechanics. Stress fractures of the bowed femoral shaft should be considered a novel subtype of AFF (Reprinted with permission from the Elsevier [65])
The authors also analyzed the stress concentration in the femoral shaft using the CT-based finite element method (CT/FEM) in the patients with AFFs or a history of AFFs who had a bowing deformity of the femur (n = 4) and control patients with thigh pain without AFFs or a history of AFFs (n = 14). All patients with either AFFs or a history of AFFs with a bowing deformity of the femur showed a marked concentration of diffuse stress on the anterolateral surface, while there were no significant findings in 13 of the 14 patients in the control group. However, the remaining patient in the control group showed a marked concentration of diffuse stress on the anterolateral surface with radiographic evidence of a bowing deformity and a focally thickened lateral cortex, similar to that which was observed in the bowed AFF group. When these patients were reclassified as either SBF (n = 5) or non-SBF (n = 13), the femoral bowing of the patients with SBF was significantly more severe in comparison to that of non-SBF patients (lateral, p = 0.0015; anterior, p = 0.0022). While no significant differences in bone mineral density or bone metabolic markers were found between the SBF and non-SBF patients, the maximum principal stress (MPS) and the tensile stress–strength ratio (TSSR) in the femoral shaft were significantly higher in SBF patients than in non-SBF patients (p = 0.0031 for MPS and p = 0.0022 for TSSR). These data suggest that the tensile stress to the femoral shaft in AFFs occurs due to the bowing deformity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








