Chapter 4 The evidence for pathoanatomical lesions
Introduction
There is no paucity of available information related to whiplash injury resulting from a motor vehicle crash (MVC), with well over 10,000 publications on diagnosis alone. Accurate estimates of recovery rates following whiplash have been inconsistent, likely due to the variety of outcome measures used and amount of time between follow-up.1–3 For instance, Barnsley and colleagues originally put forth evidence to suggest that up to 40% of people with whiplash injury will, for unknown reasons, transition from acute to persistent pain.4 However, a recent review estimates that approximately 50% of people continue to report symptoms in the long term.5 Despite discrepancies in the reported prevalence rates for long-term symptoms, some patients, females more so than males,6 will present acutely with a markedly complex clinical picture and a myriad of symptoms that can challenge even the most astute clinician. It is these patients who continue to report long-term symptoms.7
The cardinal feature of the acute and chronic condition is neck pain but can also include a variety of signs and symptoms, such as dizziness, visual and auditory disturbances, temporomandibular joint pain, photophobia, dysphonia, dysphagia, dysarthria, fatigue, cognitive difficulties (e.g. concentration and memory loss), anxiety, insomnia and even depression.8–14 Such variability underpins the challenge of building a distinct pathoanatomical diagnosis in most cases.
Whiplash injuries from MVC are defined by energy transfer to the cervical spine resulting from acceleration–deceleration forces that typically occur with rear-end (but also frontal and side) impact MVCs (Chapter 3). Such forces can, in some cases, lead to excessive non-physiological stresses on the cervical spine,15–17 and could result in injury to many disparate regional tissues.
Undoubtedly, the situation would benefit if there were quantitative radiological markers of the disorder; however, consistent observations of a salient structural lesion or lesions (that can be differentiated from normal variants in healthy individuals) with advanced imaging modalities in both the acute and persistent stages remains inconclusive.18–21
For the most part, investigations using advanced imaging (e.g. radiographic X-ray, computed tomography [CT] and magnetic resonance imaging [MRI]) have focused on various anatomical structures (e.g. osseoligamentous tissue, intervertebral discs, neural and vascular structures, and zygapophyseal [facet] joints).18–30 However, given the infrequency of abnormalities detected, the lack of prognostic value, inaccessibility of ‘clinic friendly’ measures and the high cost of advanced imaging procedures, support for an evidence-based pathoanatomical model has not, in most cases, been justified.19, 24, 25, 27, 28, 30–32 As a result, there continues to be long-standing debate about whether persistent pain is attributable to physical pathology or to mechanisms such as deliberate exaggeration and simulated gain.33–35
The aim of this chapter is to briefly describe the pathomechanics of the whiplash event, as well as summarise the available literature regarding the pathoanatomical features of acute and persistent whiplash-related pain and disability. The chapter is broken into a number of sections highlighting the proposed anatomical site(s) of injury (e.g. zygapophyseal joints, spinal ligaments, intervertebral discs, neural and vascular structures, and cervical muscles).36–38
Pathomechanics of whiplash injury
Original observations of the whiplash mechanism detailed a rapid hyperextension of the cervical spine, which created large angular displacements in the sagittal plane.40 On the contrary, current best evidence acknowledges that injury likely emerges from the inertial response of the body to the forces delivered to it, in which the head–neck complex undergoes displacement without being exposed to direct impact (e.g. the ‘S-shaped’ curve).15, 16 The consequences of either a rear-end or frontal impact could profoundly affect the cervical spine and may injure any number of anatomical tissues, such as the intervertebral discs,40 facet capsule,41–44 the anterior–posterior longitudinal, supra- and interspinous ligaments,45–47 and/or the ligamentum flavum.47
The preceding provides a brief overview of the pathomechanical features of the whiplash injury. For a more detailed description, the reader is referred to Chapter 3, which details the mechanisms of injury. The following sections highlight the available evidence regarding a pathoanatomical lesion, or set of lesions, in cases of acute and persistent whiplash, and how these injured anatomical sites may be responsible for the generation and maintenance of whiplash-related pain and disability.
Pathological lesions in whiplash injury
In a seminal study, Taylor et al. investigated cadaveric spines of individuals involved in fatal injuries compared with those who died of other natural causes.48 They were able to demonstrate a variety of injuries involving the cervical facet joints, spinal dorsal root ganglia, intervertebral discs and ligamentous tissues. Other structures have also been implicated, including injuries of the craniocervical junction, such as fractures of the dens, laminae and articular processes,49 vascular structures50–55 and other soft aqueous tissues (e.g. skeletal muscle).36, 37, 56
Zygapophyseal (facet) joint injury
The anatomical make-up of the cervical facets from C2 to C7 consists of two adjacent synovial joint surfaces enclosed by a thin capsular ligament. This flexible ligament is not capable of influencing facet joint motion; rather, its anatomical structure is dependent on the movement of the surrounding vertebrae.57 Extending between the margins of the articulating bony surfaces is a synovial fold of the inner facet capsule.56, 57
Excessive motion of articular processes, including the capsule, during whiplash loading have been identified in both cadaveric and human volunteers,16, 42, 45 and have led to two proposed mechanisms implicating facetogenic pain:
A plethora of cadaveric evidence supports strain injury to the facet capsule during whiplash loads.41, 42, 44 Normal bending of the neck joints increases capsular strain by approximately 10%, whereas whiplash loading increases peak capsule strain upwards of 40% in the lower segments (C6–C7).17, 42 Head-turned postures, which are not uncommonly reported by patients, can double the peak capsular strain during simulated whiplash,58 and may contribute to partial rupture of the capsule. Such partial ruptures have been observed in vitro to occur at strain levels lower than those observed in whiplash simulations,57, 59 suggesting that elongation of capsular fibres is potentially damaging in some subjects59 and could contribute to the transmission of pain.
Support linking facet joints in the transmission of pain following injury can be found from neuroanatomical studies that have revealed how the joints are richly innervated with Aδ– and C-fibres.60–63 Encapsulated neurons within the joint may be sensitised or excited by a gradient change in local pressure, capsular stretching and/or in response to naturally occurring pro-inflammatory agents (e.g. substance P and phospholipase A).64 Ohtori et al. further demonstrated that ongoing nociception can contribute to physiological changes in the sensitised dorsal root ganglion of rats,63 suggesting that the origins of more widespread body pain may result from massive inputs of noxious stimuli following facet joint injury.
Other animal studies have demonstrated persistent afferent discharges long after the removal of abnormal facet joint loads and prolonged stretch to the facet capsule.65, 66 Painful joint loading and capsular strain has also been shown to produce inflammatory responses in the rat spinal cord, including the development of mechanical hyperalgesia, and this cascade of events may contribute to the generation of facet-mediated pain in some following whiplash.67, 68
Significantly, the behavioural responses of animals are remarkably similar to those observed in the more ‘complex’ whiplash group, including widespread hypersensitivity,69, 70 all of which may implicate a facetogenic cause (or at least contributor) underlying central hyperexcitability; a common feature for this complex patient group.69, 70
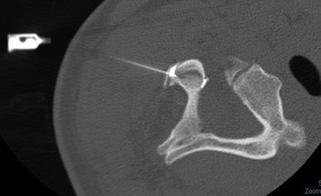
Supportive clinical evidence for a facetogenic cause (and maintenance) of persistent symptoms following whiplash is also available.71–73 Specifically, the performance of and response to level-specific, placebo-controlled diagnostic facet joint blocks were evaluated.72 The sample consisted of 68 consecutive patients referred for neck pain secondary to an MVC with a history of painful symptoms for more than three months. Individuals with a predominant headache underwent a third occipital nerve (TON) block and were excluded if they received pain relief, as the TON has a branch supplying the C2–C3 facet joint. Twenty-seven patients with pain from this segment were excluded from participating in the placebo-controlled study as the resultant pain response was likely due to anaesthetic blocks of the TON. Consequently, 41 patients underwent diagnostic blocks with either a short-acting or a long-acting local anaesthetic, followed by a second block with either normal saline or the other anaesthetic, followed by a third block with the remaining agent. The positive responders experienced complete relief with each anaesthetic and no relief with the normal saline. The results indicated a 60% prevalence of cervical facet joint pain after whiplash injury, and the most common levels affected were C2–C3 and C5–C6.72 As such, the development of diagnostic facet joint blocks and subsequent management options with radiofrequency neurotomy has been shown to temporarily reduce/eliminate persistent pain.71–73 Figure 4.1 shows a lateral radiograph and the insertion of the needle during a diagnostic facet block of the C2–C3 facet joint.
Dorsal root ganglia and nerve injury
The clinical presence of sensory disturbances, suggestive of altered pain processing, in the acute and persistent stages of whiplash, has been documented.69, 70, 74 Changes, such as local (over the cervical spine) and remote (periphery) hypersensitivity to pressure and thermal stimuli on the skin, may be explained by augmented pain processing.69, 70, 74–78 Such hypersensitive pain responses may signal the involvement of injured proximal structures (e.g. the dorsal root ganglion [DRG] and dorsal root). Direct trauma to the DRG may well affect the internal cell bodies connected with peripheral sensory nerves, and this may help explain many of the signs/symptoms for patients with a more complex clinical presentation. While speculative at this stage, pathoanatomical support for such a premise can be found from Taylor et al.,48 who found a significant prevalence of cervical DRG injury in persons involved in fatal blunt trauma to the cervical spine.
Animal studies also show physiological support underlying a potential DRG pain generator. For example, exposing anaesthetised pigs to whiplash conditions (extension, flexion and lateral flexion of the cervical spine) resulted in a reduced pressure inside the spinal cord.79 Other porcine investigations demonstrated a breach in the plasma membrane of the spinal ganglia nerve cells, consistent with injury at a cellular level following an extension-type whiplash injury mechanism.80
The segmental DRG are comprised of distinct nerve fibres responsible for relaying specific information related to proprioception, pain and temperature.81 Sato and Kikuchi demonstrated age-related morphological changes occurring in the cadaveric lumbar DRG.82 While evidence suggests these changes could influence clinical symptoms in patients with lumbar spine pathology,83–87 the impact on patients with whiplash-related pain and disability remains inconclusive.56, 67, 80, 88, 89
Another potential mechanism for producing persistent neck pain following whiplash injury involves the deformation of the cervical nerve roots. The cervical dorsal roots arise from the posterior and anterior surfaces of the spinal cord and travel anterolaterally, intrathecally, towards the intervertebral foramen. The anterolateral direction of the roots leaves them vulnerable to excessive stretching during rapid acceleration–deceleration (S-shaped curve) or lateral bending of the neck, as has been demonstrated in rear-end and side-vector impact whiplash. Deformation and injury of the nerve roots in response to extreme neck positions and changes in neural foramina diameters90–92 may represent another mechanism for producing persistent pain and disability.83, 93
It may be that, despite a lack of radiological evidence, a minor and/or major DRG and/or nerve root injury has occurred in some patients, which may, in turn, contribute to the signs of augmented pain-processing mechanisms69 and the development of persistent symptoms. Further work is warranted to investigate the potential mechanisms involved. This would necessitate, for example, comprehensive research on animal models, where lesions to various cervical structures could be controlled, and inflammatory markers and histological changes in nerve and muscle tissue could be manipulated and documented over time.
Disc injuries
Despite controversy, evidence suggests that one out of four patients with whiplash injury will have radicular symptoms in tandem with radiographic (MRI) documentation of cervical disc herniations and cartilaginous end-plate injuries.40, 94, 95
The intervertebral disc is located between adjacent vertebral bodies and consists of a central nucleus pulposus surrounded by concentric rings of annular fibres. The annular fibres are attached to the anterior–posterior longitudinal ligaments, and such an arrangement may predispose the disc to injury if the ligaments were stretched beyond their normal physiological limit, as may be the case in some whiplash events.40, 94, 95
The most common level of disc injury during frontal and rear-end impacts is the C5–C6 disc.96 Subfailure injuries have also been noted in the more cephalad discs, including C2–C3. Such findings may align with recent biomechanical research showing a greater risk of low-grade spinal cord injury during whiplash in those individuals with pre-existing spinal canal narrowing at C5–C6.96 Future research efforts should aim to correlate ligament and disc injuries for different head and neck positions, vector impact dependency and thresholds for injury before more definitive conclusions regarding the link to pain and prolonged symptoms can be drawn.
Ligament injuries
Mid–lower cervical spine ligaments
Below C2 exists a network of ligamentous tissues—the anterior and posterior longitudinal, capsular, inter- and supraspinous ligaments and the ligamentum flavum. The anterior and posterior longitudinal ligaments span the entire spine, attaching directly to the anterior and posterior surfaces of the vertebral bodies, respectively. They also merge with the annulus fibrosus of each intervertebral disc and are well innervated.97 The capsular ligaments, as described previously, envelop the facet joints.57 The interspinous ligaments are not infrequently absent but, when present, connect between adjacent spinous processes and blend posteriorly with the supraspinous ligament.56 The highly elastic ligamentum flavum is poorly innervated98 and joins the adjacent lamina bilaterally.99
Spinal ligaments possess mechanoreceptive and nociceptive nerve endings that, if injured, may contribute to sensorimotor deficits related to abnormal neck mobility, altered proprioception and transmission of pain.11–13, 100 However, the lack of available evidence to provide conclusive evidence linking mid–lower cervical ligament disruption and symptoms in whiplash is noted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree