Health economics
The study of how scarce resources are allocated amongst alternative uses for the care of sickness and the promotion, maintenance and improvement of health, including the study of how health care and health-related services, their costs and benefits and health itself are distributed amongst individuals and groups in society
Quality-adjusted life-years (QALYs)
A quantitative measure of life (in years) adjusted by the impact of an intervention. Adjustments are made on a yearly basis according to a 0–1 scale, with 0 indicating death and 1 indicating 1 year of perfect health
Incremental cost-effectiveness ratio (ICER)
A quantitative measure of the additional benefits gained from a new therapeutic intervention compared to an alternative or standard intervention. The formula for the incremental cost-effectiveness ratio (ICER) is
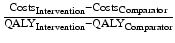
Direct costs
All resources that are consumed in the provision of a health promotion programme. These may be incurred by the health-care provider, community or individuals
Indirect costs
These relate to the losses to society incurred as a result of participating in the programme, such as the impact on production, domestic responsibilities and social and leisure activities
Intangible costs
These relate to issues such as anxieties and impact on quality of life resulting from participation in the programme. These are difficult to measure and value and are often not included in the construction of the cost profile of an economic evaluation
Willingness-to-pay threshold
A subjective and explicit threshold determined by a health-care provider to state the maximum amount they would be willing to pay to receive a particular benefit. This is an indicator to the value to them of that commodity. Typical cost-effectiveness thresholds are $50,000–100,000/QALY in the USA and $32,852–49,278/QALY in the UK
Markov model
A type of decision model allowing for transition between health states over time. The probability of each transition is modelled with the cost and utility of each outcome, with quality-adjusted life-years accumulated each year according to the health state of the patient
Patient-reported outcome measures (PROMs)
Validated instruments such as questionnaires and scales used quantify a patient’s judgement of their disease before and after surgery. PROMs can be generic and hence widely applicable or specific to particular health problems or population. Generic PROMs include SF-36 and EQ-5D, and specific PROMs include Oxford Knee Score
Sensitivity analysis
There is potential for a large variation in the estimate of cost-effectiveness due to methodological factors, variation in the estimates of cost and effects, transferability and validity of results from different patient groups and the impact of extrapolation of observed events over time. Sensitivity analysis is a process through which the robustness of an economic model is assessed by examining the changes in results of the analysis when key variables and assumptions are varied over a specified range
The costs and benefits of TKA do however vary substantially between patient groups. Savings are greater in the youngest patients undergoing knee arthroplasty $158,110 in 2008 dollars ($160,582 in 2015 dollars), but direct medical costs exceed societal savings at a patient age of 70 years at the time of the index procedure. Savings are greater in patients attending high-volume centres (>200 TKA/year) and those stratified as ‘low risk’ based on age, poverty and medical co-morbidities [14]. Although evidence and intuition would suggest that older patients have higher medical costs associated with TKA [15], one study suggests that compared to nursing home placement of nonagenarian patients in the UK, the lifetime cost in TKA is cheaper [16]. Finally, the probability and rate of return to employment affect the economic benefit of TKA – conservative management is superior to surgery in those patients who would lose than 18 workdays/year due to osteoarthritis [13]. In those of working age, 98 % of patients return to work after TKA [17].
9.4 Cost-Effectiveness of TKA
Costs can be calculated from either a societal or health-care payer perspective. By calculating ‘value’, one considers the financial investment of TKA and its cost-effectiveness simultaneously. The marginal and incremental cost-effectiveness ratio (ICER) relates the costs of a treatment to its benefits from a patient’s perspective. If there is a willing payer, TKA can ultimately only be justified if it improves disease symptomatology and provides patients with satisfaction and function. Patient-reported outcome measures (PROMs) such as the Oxford Knee Score (OKS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire, the Intermittent and Constant Osteoarthritis Pain (ICOAP) and Osteoarthritis Score-Physical Function Short Form (KOOS-PS) and generic instruments such as Short Form-36 (SF-36), SF-12, SF6D and EuroQuol-5D (EQ-5D) questionnaires are commonly used to measure quality of life in relation to gonarthrosis. The balance between the cost of TKA and its impact on quality of life is mostly estimated in terms of monetary units per gained quality-adjusted life year (QALY). It allows for a patient-related interpretation of benefit as well as providing a comparison of its cost-effectiveness with alternative, conservative management. Individual service providers may have specific thresholds for when a treatment becomes ‘cost-effective’. This threshold is influenced by budget constraints, social willingness to pay, the value of health used elsewhere in the public sector, data on cost-effectiveness of existing services and past decisions. In the UK, a treatment that can enhance quality of life at a cost of less than $49,278/QALY (excluding end-of-life treatment and emergencies) is deemed viable [18]. For developing countries, the World Health Organization has proposed a cost-per-QALY threshold of 3 times the per capita gross domestic product to guide their health-care resource allocations. There is no explicit threshold for willingness to pay in the USA, though it is imputed between $50,000 and 100,000 [19] (Fig. 9.1; Table 9.2).
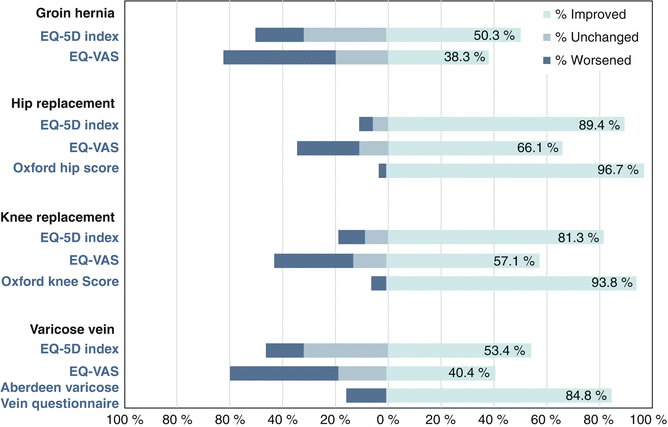
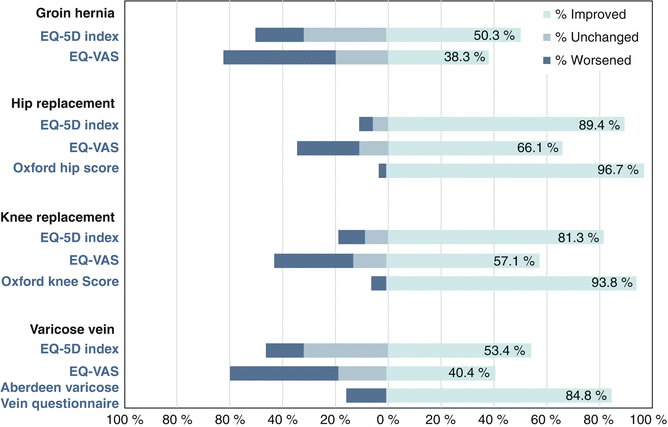
Fig. 9.1
PROMs (patient-reported outcome measures) for surgery in the United Kingdom (2014) [1]. Since 2009, pre- and post-operative PROMs have been collected for four elective procedures in the UK: hip surgery, knee surgery, hernia repair and varicose veins surgery. Generic PROMs (EQ-5D and EQ-VAS) are collected along with procedure specific PROMs, except for groin hernia repair for which there is no accepted instrument. Almost 94 % of patients have improved functional scores after knee arthroplasty (Reproduced from Health and Social Care Information Centre UK, 2015) EQ EuroQol, VAS Visual Analog Scale
Table 9.2
Approximate Cost/QALY (quality-adjusted life years) gained for surgical procedures in the UK
Procedure | Cost/QALY gained (2015 dollars) |
|---|---|
Total knee arthroplasty | $3,323 (21) |
Total hip arthroplasty | $2,170 (21) |
Groin hernia repair | $2,974 (20) |
Neurosurgery for malignant intracranial tumours | $455,250 (1998 estimates converted to 2015 estimates) |
Kidney transplantation | $273,150 (1998 estimates converted to 2015 estimates) |
Hospital haemodialysis | $50,078 (1998 estimates converted to 2015 estimates) |
In a European study, the cost per QALY of TKA was calculated at an overall ratio of €1,795. Again, cost-effectiveness analysis is particularly sensitive to the age of the patient. While patients <60 years yielded an incremental cost/benefit relation of $1,622 (in 2015 dollars), each QALY in patients >70 years costs $4,012 (in 2015 dollars), due to the assumption of a smaller rest-of-life expectancy. The clinical benefit was comparable amongst all patients, with increases of 31–40 % in quality of life scores [22]. An American economic model used a lifetime horizon to calculate the overall ratio at $18,300/QALY ($20,182 in 2015 dollars), increasing to $28,100 in 2009 dollars ($30,990 in 2015 dollars) in high-risk patients due to perioperative morbidity and failure rates [14]. This determined a clinical difference on the WOMAC questionnaire of >40 points.
The cost-effectiveness of TKA is also sensitive to hospital volume. For low-risk patients in a high-volume centre, the cost-effectiveness findings for TKA were $9,200/QALY ($10,110 in 2015 dollars). This dominated strategy (i.e. lower costs and increased health benefit) compared to performing TKA on high-risk patients in low-volume centres, which provided a ratio of $107,000/QALY in 2008 dollars ($117,584 in 2015 dollars) [14]. Other economic evaluations have estimated the cost of TKA for arthritis (82 % OA, 16 % rheumatoid arthritis, 2 % other) over 1 year to be $14,000/QALY in 2006 dollars ($16,430 in 2015 dollars) averaged across all patient age groups, though this only included surgeon- and hospital-related costs [23].
A prospective study of the cost-effectiveness of TKA in 212 North American patients used WOMAC pain and functional scales, direct and indirect costs calculated from US Medicare reimbursement fees and estimates of productivity losses for patients and relatives. Although limited by its design (a time horizon of just 6 months), 80 % reported improved symptoms. The mean total cost per TKA was $24,435 in 2014 dollars ($24,420 in 2015 dollars) – compared to the hypothetical costs of not performing TKA of $4,303 ($4300 in 2015 dollars). The ICERs for WOMAC improvement at 6 months were $33,345 ($33,325 in 2015 dollars) to achieve the authors’ preset minimum clinical important difference of 20 points [24].
Patients with walking impairments from OA run a risk of early death that is 1.5 times higher than the general population. This cohort has a higher chance of concurrent medical co-morbidities (most commonly Ischemic heart disease, chronic respiratory conditions, diabetes and cerebrovascular disease) [25]. American (Medicare) patients receiving TKA show nearly half the risk of death after 7 years compared to OA patients not receiving TKA [26]. Patients operated on earlier in the course of their functional decline report better immediate and midterm postoperative outcomes. Ultimately, delaying surgery in patients with end-stage gonarthrosis is not cost-effective [14]. Still, only a minority of patients with appropriate indications for TKA actually undergo the procedure – perhaps as low as 13 %. There is a particular disparity in women who are more likely to report symptomatic knee OA but less likely to undergo arthroplasty surgery [27].
In conclusion, while TKA is robustly a highly cost-effective procedure for the management of end-stage gonarthrosis, patient risk level and particularly hospital volume play an important role. The established willingness-to-pay threshold is also affected by the outcome metrics used to measure clinical difference and is individual to the service provider. However, multiple studies have shown that timely TKA remains cost-effective compared to not performing surgery regardless of setting, patient risk and postoperative mortality.
9.5 Cost-Effectiveness of Unicompartmental Knee Arthroplasty
A large proportion of patients who are eligible for TKA may be eligible for unicompartmental knee arthroplasty (UKA). The indications and contraindications for UKA remain controversial [28]. There is a consensus that patients with significant symptoms corresponding with unicompartmental medial or lateral arthritis, with functional ipsilateral collateral ligaments and anterior cruciate ligament, and full-thickness cartilage in the opposite compartment are suitable for UKA. The potential advantages of UKA over TKA are preservation of bone stock, less invasive surgery, minimal blood loss, fewer complications, more rapid rehabilitation and greater physiological function [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








