Fig. 2.1
Anteroposterior (a), lateral (b), and axial (c) radiographs of a 71-year-old woman with severe idiopathic knee osteoarthritis
Rolauffs et al. identified the presence of angular characteristics of spatial chondrocyte organization and species-specific remodeling processes correlating with OA onset [5]. The appearance of distinct angular and spatial patterns between neighboring chondrocytes can identify the onset of distant OA prior to microscopically visible tissue damage and possibly before clinical onset. With further development, this concept may become suitable for the diagnosis and follow-up of patients susceptible to OA.
One of the mechanisms of cartilage degradation in OA is enzymatic proteolysis of the extracellular matrix by metalloproteinases. MMP-1, produced by chondrocytes and synovial cells, is a major proteinase of the matrix metalloproteinase (MMP) family [6]. Wassilew et al. evaluated a subset of MMPs and proinflammatory cytokines within the synovium, from traumatic knee disorders (TKDs) and nontraumatic OA [7]. Quantitative analysis showed no significant differences in the expression levels of MMPs, interleukin-1 beta, or TNF (tumor necrosis factor)-alpha messenger with ribonucleic acid (RNA) between the synovial tissues of patients with OA and TKD, but C-reactive protein (CRP) level was significantly increased in the OA group. A significant correlation was also seen regarding the gene expression levels between MMP-1 and MMP-3, as well as between CRP and MMPs tested. Furthermore, significant relations between TNF-alpha and MMP-1 plus MMP-3 were observed in the OA synovial tissue. The level of TNF-alpha in the synovial tissue correlated with the time after injury as well as chondral damage in patients with TKD. This study showed similar changes in the inflammatory patterns of synovial tissue of TKD and OA suggesting a likely disease progression. Moreover, the correlation between CRP and MMP expression levels indicated their essential role in joint degeneration in synovial tissue of primary OA patients. TNF-alpha could provide a factor to quantify individual risk for the development of OA.
The study reported by Lepetsos et al. evaluated the association of MMP-1 gene -1607 1G/2G (rs1799750) polymorphism with primary knee OA in the Greek population [6]. There was no significant association between MMP-1 -1607 1G/2G polymorphism and knee OA, in crude analysis; however, after multiple logistic regression analysis, 1G/2G was associated with reduced odds of knee OA by 75 % in males, compared to genotypes 1G/1G + 2G/2G, adjusting for age and BMI (body mass index). The study showed that MMP-1 -1607 1G/2G (rs1799750) polymorphism might be a risk factor for knee OA susceptibility in the Greek population.
In a meta-analysis, Rodríguez-Fontela et al. assessed candidate genes for association with OA, identifying promising genetic factors and assessing the candidate gene approach in OA [8]. Two candidate genes, COL11A1 and VEGF, were significantly associated with OA.
Strong familial aggregation and heritabilities have been reported for knee OA. Candidate gene studies and genome-wide linkage studies have identified genes in the bone morphogenetic pathway (e.g., GDF5), the thyroid regulation pathway (DIO2), and the apoptotic pathways as involved in genetic risk of large joint OA. Genome-wide association studies have reported structural genes (COL6A4/DVWA), inflammation-related genes (PTGS2/PLA2G4A), and a locus on chr 7q22 associated with knee OA [1].
A number of phenotypes of knee OA have been identified and validated based on similarities in clinical patient characteristics [9]. Cluster analysis identified five phenotypes of knee OA patients: “minimal joint disease phenotype,” “strong muscle strength phenotype,” “severe radiographic OA phenotype,” “obese phenotype,” and “depressive mood phenotype.”
Genetic factors play an important role in the pathogenesis of knee OA, but which knee structural changes mediate this is unclear. The study of Pan et al. aimed to describe the differences in knee structural changes over 8–10 years between offspring having at least one parent with total knee arthroplasty (TKA) for severe primary knee OA and controls with no family history of knee OA [10]. One hundred and fifteen offspring (mean age 45 years) with a family history of TKA for severe knee OA were compared with 104 (mean age 46 years) controls. T1- or T2-weighted fat-saturated magnetic resonance imaging (MRI) was performed, respectively, to evaluate knee cartilage defects, bone marrow lesions (BMLs), meniscal extrusion, and tears at baseline and 10 years. Multivariate logistic regression model was used to adjust for potential confounders. Offspring had a greater increase in cartilage defect score and meniscal extrusion score over 10 years and a greater increase in meniscal tear score over 8 years in the medial but not in the lateral tibiofemoral compartment. Changes in BMLs over 8 years were not different between the two groups. These associations were independent of potential confounders and strengthened after further adjustment for each other. With the exception of BMLs, offspring with a family history of knee OA have a greater risk of increases in multiple knee structural abnormalities in the medial tibiofemoral compartment suggesting pleiotropic familial effects [10].
Little is known about the temporal evolution of pain severity in persons with knee OA. Collins et al. sought to describe the pain trajectory over 6 years in a cohort of subjects with radiographic, symptomatic knee OA [11]. Collins et al. found that knee pain changed a little, on average, over 6 years in most subjects. These observations suggested that knee OA is characterized by persistent rather than inexorably worsening symptoms.
Barret et al. studied the correlation of radiographic patterns and clinical manifestations of symptomatic idiopathic OA of the knee [12]. A retrospective review of weight-bearing radiographs of 2,197 knees in 1,894 patients with symptomatic idiopathic OA revealed six different patterns of tibiofemoral deformity. Five of these patterns expand the taxonomic concepts of Ahlback; the sixth, “nonproliferative,” was previously undescribed. It was seen exclusively in patients with varus disease and was characterized by a lack of reactive bony changes. Knees with degenerative changes in the medial compartment constituted the majority of cases (63 %). The average age of patients was 72 years for those with varus disease, 79 years for those with valgus disease, and 84 years for those with patellofemoral arthritis (PFA). Bilateral involvement was common only in patients with PFA (79 %), suggesting a developmental cause for this subset. There was a female predominance in valgus and patellofemoral disease.
2.2.2 Post-traumatic Osteoarthritis
Post-traumatic OA follows different types of injuries to the knee joint such as tibial plateau fractures, anterior cruciate ligament (ACL) injuries, and surgical total meniscectomy (Fig. 2.2). Gouttebarge et al. [13] analyzed the prevalence of OA in former elite athletes from team and individual sports. A systematic review of observational studies was conducted. Based on three categories of keywords (and synonyms), a sensitive search strategy was built in order to search MEDLINE and SPORTDiscus from 2000 to 2014. Knee OA ranged from 16 to 95 %. Prevalence rates of general, lower limb, or hip/knee OA ranged from 1 to 59 %. The study showed that prevalence of OA, especially in their lower limbs, seems to be high among former elite athletes from team and individual sports compared to the general population and other occupational sectors [13].
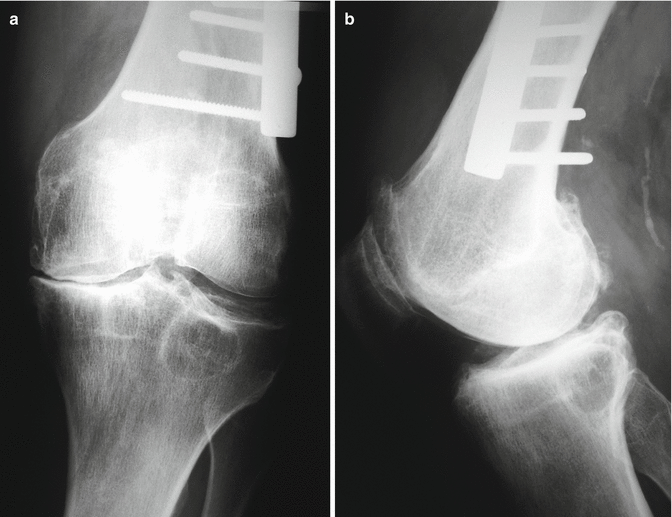

Fig. 2.2
Anteroposterior (a) and lateral (b) radiographs of a 47-year-old man showing post-traumatic knee osteoarthritis due to an old supracondylar femoral fracture healed in varus
In a study reported by Rodríguez-Merchán et al., 25.4 % of patients showed symptomatic OA changes in the knee 15 years after ACL reconstruction using the central-third patellar bone-tendon-bone (BPTB) autograft [14].
2.2.3 Hemophilic Arthropathy
Hemophilia patients suffer from a congenital clotting defect that makes them prone to multiple intra-articular hemorrhages (hemarthroses). These may be spontaneous or caused by minimal trauma and usually affect the knees, ankles, and elbows. Such recurrent hemarthroses of the knees cause joint degeneration (hemophiliac arthropathy) at a very early age (20–30 years) [15] (Fig. 2.3). The only way to combat this degeneration – although it cannot be completely prevented – is through primary prophylaxis before the first hemarthrosis takes place (or after 1–2 hemarthroses) [16]. It is essential to protect the joints of hemophiliac patients through the use of primary prophylaxis. At present, 70–80 % of patients do not receive adequate treatment due to financial and economic constraints. Hemophilia patients with very painful and/or incapacitating knees as a result of the abovementioned degenerative arthropathy frequently require TKA if there is to be a significant improvement in their quality of life [17, 18].
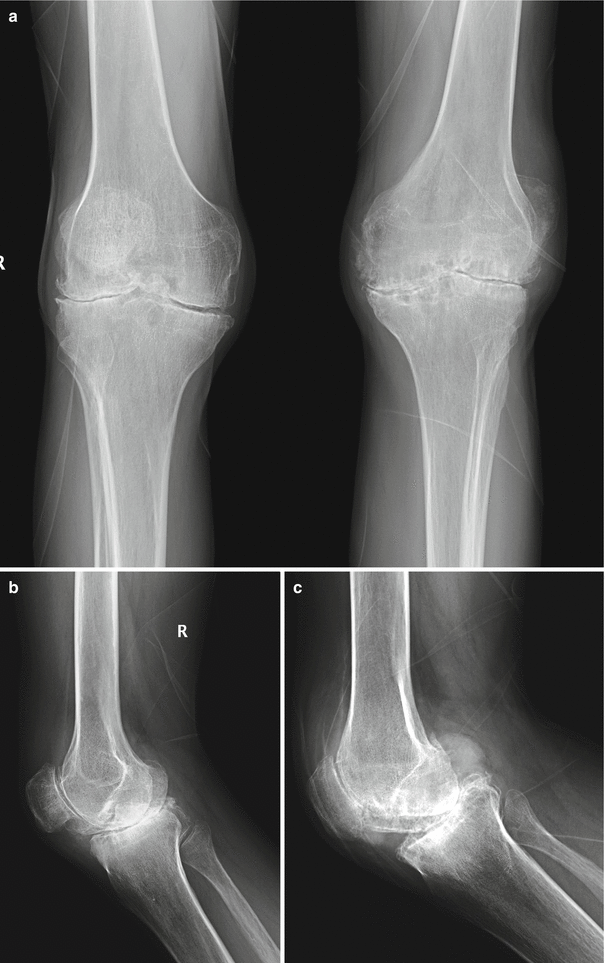

Fig. 2.3
Anteroposterior (a) radiograph of both knees and lateral views of the right knee (b) and left knee (c) of a 37-year-old man with hemophilia
2.2.4 Inflammatory Osteoarthritis
Inflammatory arthritis of the knee is reviewed in depth in Chap. 3. However, it is important to emphasize that pigmented villonodular synovitis (PVNS) is a synovial disorder in which the hypertrophic synovium (Fig. 2.4) may lead to joint destruction and activity limitation [19]. Synovectomy (open or arthroscopic) may delay the need of TKA in patients with PVNS [20, 21]. Psoriatic knee arthritis may also cause severe joint destruction due to hypertrophic synovitis (Fig. 2.5).
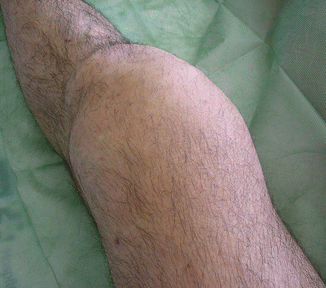
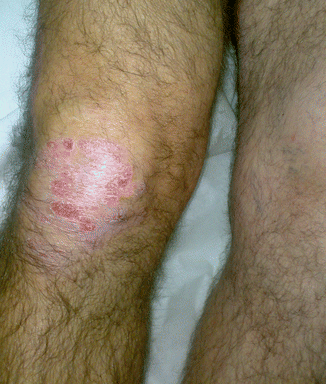

Fig. 2.4
Clinical view of a 46-year-old man with intense knee pigmented villonodular synovitis

Fig. 2.5
Clinical view of a 51-year-old man with moderate knee synovitis associated with psoriasis. Note the typical skin lesion of psoriasis
2.3 Patterns of Disease
2.3.1 Disease Stage
Zhang et al. have tried to identify metabolic markers that can classify patients with OA into subgroups [22]. Two distinct patient groups, A and B, were clearly identified in the 80 patients with OA. Patients in group A had a significantly higher concentration on 37 of 39 acylcarnitines, but the free carnitine was significantly lower in their synovial fluids than in those of patients in group B. The latter group was further subdivided into two subgroups, that is, B1 and B2. The corresponding metabolites that contributed to the grouping were 86 metabolites including 75 glycerophospholipids (6 lysophosphatidylcholines, 69 phosphatidylcholines), 9 sphingolipids, 1 biogenic amine, and 1 acylcarnitine. The grouping was not associated with any known confounders including age, sex, BMI, and comorbidities. The possible biological processes involved in these clusters are carnitine, lipid, and collagen metabolism, respectively. The study demonstrated that OA consists of metabolically distinct subgroups. Identification of these distinct subgroups will help to unravel the pathogenesis and develop targeted therapies for OA [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








