Fig. 3.1
Normal aspect of the ACL through the anterolateral and anteromedial portals and the knee close to 90° of flexion. The synovial vasculature is visible, as well as the large tibial insertion and the twisted narrower body. The bundle structure can sometimes be apparent. The femoral insertion is more difficult to visualize when the ACL is not ruptured (Kundnan)
3.1.3 Injury Mechanism and Patterns
A large number of ACL ruptures are isolated and occur through non-contact injuries. Understanding the causative mechanism might help determine methods for prevention. Anterior tibial shear forces with the knee close to full extension during landing are considered to be the main cause for noncontact ACL injuries. These forces are mainly determined by quadriceps contracture, as demonstrated by both experimental and in vivo studies [21, 22].
When the knee is at less than 30° of flexion, the hamstrings pull no longer exhibits a protective effect on anterior tibial translation. As the knee is flexed, the ACL is unloaded. Internal tibial rotation acts synergistic and increases the combined knee loading, whereas valgus or valgus stress does not significantly increase the loading of the ACL [23].
Although the ACL is also a restraint to forced valgus, this function was considered to be mostly exhibited after the medial collateral ligament (MCL) is torn. Recent cadaveric studies have shown that the ACL strain levels that can be reached during landings are lower than those needed to produce injury to the MCL [24]. It is therefore likely that valgus stress plays a role in the injuring pattern in combined triad injuries such as MCL, ACL and medial meniscus. Nevertheless, internal tibial torque combined with knee valgus during pivot landings loads the ACL and especially the AM bundle even before the MCL is torn [25].
In addition, compression and posterior directed ground reaction forces during landing further contribute to increase the ACL loading [26]. One compression and internal torsion cadaver study showed that the ACL failed when these motions were exaggerated. However, after the ligament was torn, the models exhibited abnormal movement characterized by external tibial rotation and knee valgus [27]. This might explain the descriptive findings associated with noncontact injuries. Video analysis of ACL injuries in team handball confirmed these experimental determinations and observed two prevalent mechanisms. The most common was described as a plant and cut movement with the knee extended and supplemental valgus and tibial rotation. The second was forced valgus and external rotation while landing on a single limb, also with the knee close to full extension [28].
Women are at higher risk for ACL tears from noncontact injuries but the contributing factors are ill defined. Females have been shown to have increased tibial rotation and decreased hamstrings activity, which might contribute to this gender difference [29]. They have smaller ACL cross-sectional dimensions and increased lateral tibial slope, which produce greater strains on the AM bundle during pivot landings [30]. There is also an increased tibial plateau slope in ACL injured knees but imaging studies are inconsistent to determine a cut-off value [31].
Associated meniscal, chondral and bone bruises are common in patients with ACL tears. Abduction and multiplanar loading seem to be directly related to bone lesions of the tibial plateau [32]. Most ACL ruptures are complete, including both bundles. However, an observation by Zantop et al. has found up to one-quarter of the tears to be of a single fascicle. The rupture pattern of the ACL predominates at the femoral insertion, although in 44 % of cases the AM and PL bundles did not rupture at the same location. The remnant stump is thus mainly attached to the tibial insertion and can have various shapes and sizes. The longer the time from injury to reconstruction, the more absent and faded the tibial remnant is found (Figs. 3.2 and 3.3). Furthermore, reconstructed ACLs tear differently depending on the surgical procedure [33].
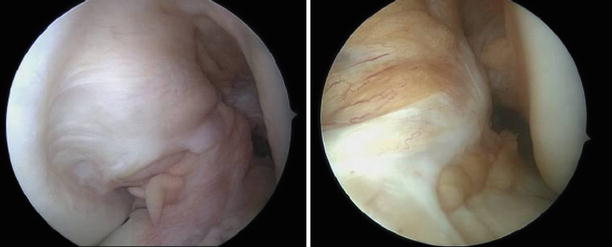
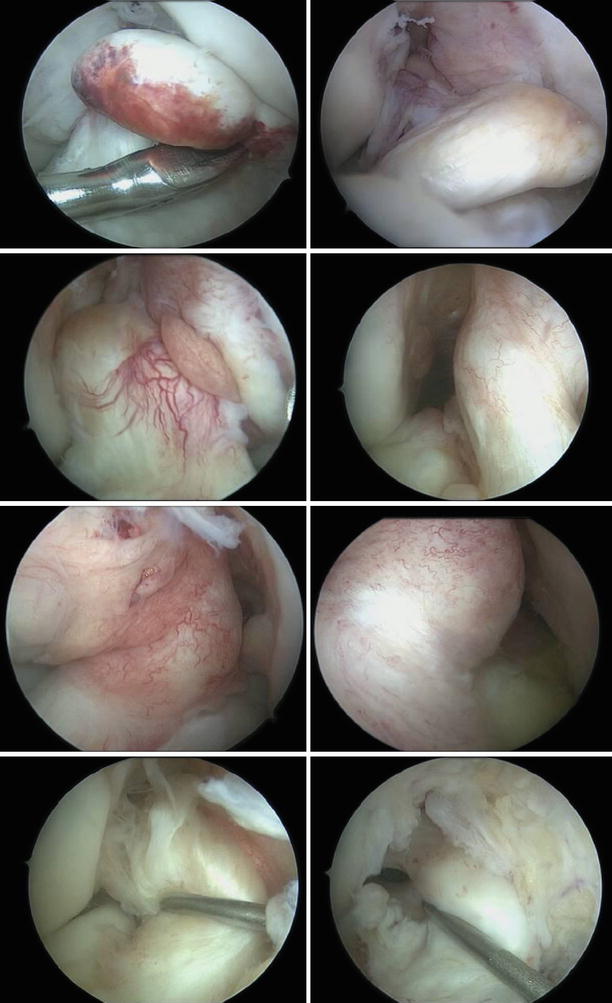
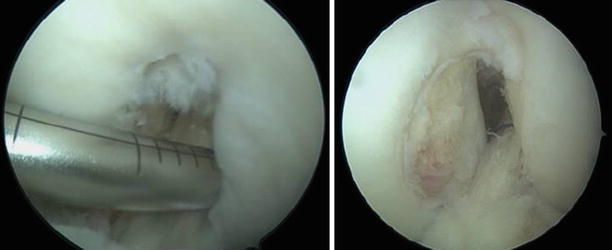


Fig. 3.2
Different shapes and sizes of the ACL remnants: from totally absent to ganglia shaped, adhered to the posterior cruciate ligament (PCL) and covered in synovia and ligaments with scar continuity

Fig. 3.3
A narrow notch can predispose to ACL ruptures but it is unclear whether this is caused by impingement or by association with smaller ACL sizes [34]
3.2 Diagnosis and Indication for Surgery
The ACL tear should be diagnosed by a thorough medical history, clinical examination and imaging studies. However, the clinical proof of anterior-posterior instability is the most common and important finding. The majority of ruptures are complete, although partial (single bundle) are also encountered but are more difficult to identify.
Acute presentations follow a recent traumatic event, most commonly during sports and, as shown earlier, as a non-contact injury. When this is the case, the knee is usually painful and difficult to examine. The patients can sometimes accurately describe the femur abnormally moving away from the tibia with spontaneous reduction. A ‘pop’ can also be heard. Swelling most commonly accompanies acute presentations and is caused by hemarthrosis. This is not mandatory and can also occur with osteochondral fractures. The overall acute clinical picture is determined by associated meniscal and chondral lesions [35]. The presence of acute swelling and suspected hemarthrosis no longer has indications for aspiration or diagnostic arthroscopy. Noninvasive imaging studies such as Magnetic Resonance Imaging (MRI) can offer a much better understanding of the lesions and prepare for definitive operative treatment. The emergent standard radiographic examination almost always fails to identify any lesions and stress x-rays are practically never performed routinely.
Isolated ACL tears can be interpreted as simple sprains and further referral to a specialist or MRI examination is not performed. After the initial acute phase the patient returns to daily activities. Subjective giving way is not always encountered during the daily routine but is more prevalent when the patient attempts to return to sports. This way, many months can pass until definitive diagnosis is made. When the ACL tear becomes chronic, the patient usually presents to the orthopedic specialist with due to recurrent instability or secondary chondral and meniscal lesions.
Anterior tibial translation with the knee flexed to 90°, known as the anterior drawer test, is the easiest clinical examination method that can be performed to determine instability. It is very specific but has a relatively high rate of false negative determinations. When this test is performed at only 20° of flexion, the test is known as Lachman and is the most reliable and sensitive clinical examination than can be routinely performed for ACL insufficiency [36]. Probably the most challenging part of performing these tests is being able to have the patient completely relax the hamstrings and quadriceps muscles. It is recommended to have the patient supine and perform gentle but firm maneuvers, especially in the acute setting, when pain can seriously and adversely influence the examination. Compared to the acute presentation, chronic ACL insufficiency usually leads to increased laxity and more pregnant anterior tibial translation [37]. The amount of anterior translation is as important as the firmness of the end point. A complete chronic tear should not have a clearly perceptible stop on anterior drawer or Lachman tests. Based on the absolute and relative (compared to contralateral) amount of translation and the presence of a firm end point, the positive anterior drawer and Lachman tests are evaluated by three severity grades: ‘+ to +++’.
As easy and useful these tests are, they rely on the examiners clinical abilities, experience and discernment. The KT-1000 (MedMetric, San Diego, USA) and the subsequent KT-2000 are the most widely accepted and used instrumented measures of antero-posterior knee laxity. A determined manual maximal difference of 3–4 mm compared to the contralateral side, especially when the absolute value is over 10 mm is a definitive confirmation of ACL insufficiency [38, 39].
The tibial plateau anterior translation also has a rotational component. The lateral plateau is more mobile as the knee is flexed. When the ACL is insufficient and the knee is flexed from near full extension to 30° of flexion, while simultaneously applying compressive and valgus loads, the anterior tibial subluxation is reduced. This can be clinically evaluated as the pivot shift test and gives a more accurate determination of knee stability during dynamic function. When performed under anesthesia it is both pain free for the patient if positive and much more sensitive. However, these particularities limit its use for diagnostic purposes. Jakob et al. described the three levels grading system currently used in practice mainly to evaluate posterolateral corner integrity and outcomes after ACL reconstruction [40]. Residual pivot shift is related to persistent subjective knee complaints, poor clinical outcomes and progression of osteoarthritis [41, 42]. Quantitative determination of the pivot shift has been recently attempted by determining spatial position and acceleration of electromagnetic sensors with promising results [43].
The MRI is the imaging modality of choice in patients with ACL injuries. It can identify tears with up to 95 % accuracy. In addition, it gives details on menisci, bone bruising, possible cartilage lesions, size and orientation of the native ACL and its insertions, thickness of the patellar and quadricipital tendons. High magnetic field machines produce better resolution, which is especially helpful for partial tears. Complete tears are seen as total discontinuity of the fibers. Incomplete tears are focal hemorrhage, non-homogenous hyperintense interruptions which appear to still have some fibers in continuity. Sagittal T2 acquisitions are the most important series to be examined. However, the coronal plane obliquity of the ligament gives a distorted and incomplete aspect of all ACL fibers on orthogonal series. This is why the standard knee protocols have a dedicated sagittal oblique acquisition. To clarify even further the state of the entire ACL fibers, a coronal oblique series can be added, parallel to the ACL in the sagittal plane. Partial tears can sometimes be misleading to identify on MRIs performed in the acute phase (Figs. 3.4 and 3.5).
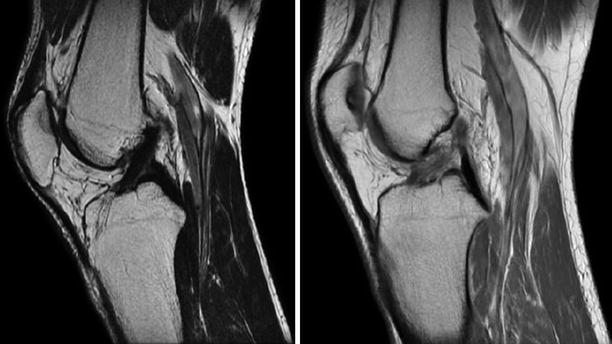
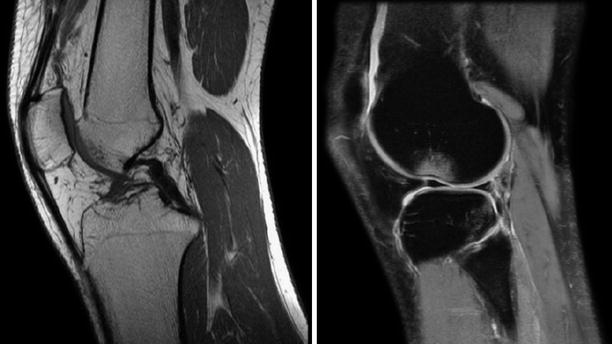

Fig. 3.4
Sagittal oblique images obtained with a 1.5 T machine depicting a normal, continuous, homogenous, hypointense ACL and a torn, hyperintense, discontinued in the proximal part ACL respectively

Fig. 3.5
Indirect signs associated with ACL tears: a curled PCL due to anterior tibial translation and lateral femoral condyle and tibial plateau bone bruises [44]
In our clinic we treat both professional and recreational athletes whom had injured their ACL during sports activity. The ACL repair is usually done at 4–8 weeks after the injury. A subsidence of the acute posttraumatic inflammation is recommended, and this period will vary between cases. An absolute connection between early surgery and postoperative arthrofibrosis has not been demonstrated. Some studies found patients operated in the first 3 weeks had an increased incidence of arthrofibrosis [45] while others showed that early reconstruction (during the first 2 weeks) led to sooner return to sports and better clinical and laxity testing results [46]. Additional factors such as patient’s predisposition to excessive connective tissue formation, associated lesions and the overall magnitude of the injury as well as the postoperative rehabilitation regimen play a considerable role in the surgical outcome. On the other hand, concomitant cartilage damage and delayed reconstruction are major risk factors for inferior outcome after ACL reconstruction [47].
The main indication for ACL reconstruction is symptomatic and clinically evident antero-posterior knee laxity in the absence of advanced joint degeneration. Associated ligamentary laxities need to be excluded or identified prior to planning for reconstruction. PCL tears can also produce anterior tibial translation but with a firm endpoint and sagging. Unaddressed MCL ruptures will give exaggerated anterior movement of the medial tibial plateau that could be confused for ACL insufficiency. Chronic MCL tears will not provide adequate support for valgus loads and will lead to ACL reconstruction failure. Undiagnosed posterolateral corner insufficiency can lead to poor clinical outcomes, persistent positive pivot shift and ACL reconstruction failure.
3.2.1 Outcomes After ACL Reconstruction
ACL reconstruction allows the patients to return to sporting activities and improves the functional outcomes [48]. However, one study found that half of patients operated 10–20 years ago, showed signs of osteoarthritis (OA) with associated pain and functional impairment. The same authors found inconclusive evidence to support the protective role of repair or reconstructive surgery of the ACL or meniscus against osteoarthritis development [49]. A comparison of conservative and reconstructive ACL surgery found similar physical activity levels for both groups at 11 years follow-up. ACL reconstruction led to higher stability but also increased risk for osteoarthritis. Nonetheless, the risk of secondary meniscal tears is reduced after reconstruction, which indirectly reduces the negative effects of OA [50]. The extraarticular augmentation procedure from the Rizzoli Institute of Bologna, Italy only demonstrated progressive joint narrowing for patients having concomitant medial meniscal surgery [51]. A retrospective evaluation performed in Pittsburg identified an almost 40 % incidence of radiographic OA approximately 8 years after ACL surgery. Prior medial meniscectomy, 2 or greater medial chondrosis and obesity were the best predictors for OA development [52]. An analysis of the MOON (Multicenter Orthopaedic Outcomes Network) cohort found patients were able to perform sports function at 6 years, but the activity level continued to decline from baseline evaluation. In addition, lateral meniscus treatment, allograft, smoking and obesity were negative predictors for long-term outcomes [53]. More than a 3-fold increase prevalence of OA was found after ACL reconstructed knees compared with the contralateral. The prevalence of OA was similar between the B-PT-B and hamstrings grafts. A meniscus resection was also a strong predictor of symptomatic degeneration [54]. In the Swedish ACL register, older aged patients (over 40 years) recorded lower preoperative KOOS scores. These patients exhibited the greatest improvement in for all subscales up to 5 years, even though they had more cartilage and meniscus injuries and longer intervals from injury to reconstruction [55]. Fourteen years after operative or nonoperative management of ACL injury, the reconstructed cohorts had less need for subsequent meniscal surgery and less decline of functional scores. The difference in pivot-shift results was not significant, as well as final outcome scores (Tegner – Lysholm, International Knee Documentation Committee – IKDC) or the rate of radiographically evident degeneration [56, 57].
3.3 Skeletal Immaturity
Over the years attitudes have changed towards favoring early surgical treatment as opposed to conservative management. Clear evidence shows that the same correlations from the adult populations exist in the skeletally immature patients regarding increase meniscal and chondral injuries with delayed treatment. This is concerning because studies have shown that, regardless of knee stability obtained after ACL reconstruction, meniscectomy accelerates degenerative joint changes [58]. Lawrence et al. [59] found that out of 70 young patients who underwent surgical reconstruction of an acute ACL tear more than 12 weeks after the injury more were noted to have a significant increase in irreparable medial meniscal tears and lateral compartment chondral injuries (odds ratio 11.3) at the time of reconstruction. When a subjective sense of knee instability was present, this association was even stronger (odds ratio 11.4). Dumont et al. [60] performed a retrospective chart review of 370 pediatric patients undergoing primary arthroscopic ACL reconstruction. They identified that index ACL reconstructions after more than 150 days since injury have a higher rate (odds ratio 1.8) of medial meniscus tears than those treated earlier. Increased age (odds ratio 1.6) and weight (odds ratio 2.2) are independently associated with a higher rate of medial meniscus tears. Patients with ACL tears and a medial or lateral meniscus tear are more likely to have a chondral injury in that particular compartment than those without meniscal ruptures.
Regarding the most appropriate graft choice there is growing support in favor of autologus hamstrings (semitendinosus and gracilis) and concern with bone – patellar tendon – bone since the bone plugs have led to premature physeal ossifications in animal models. Gebhard et al. [61] analyzed 28 patients who underwent ACL reconstruction with hamstrings, 16 patients with bone-patella-bone, 12 patients with quadriceps grafts and 12 patients with facia lata. The mean follow-up was 32 months. Neither leg length discrepancy nor angular deformities were noted. None of the four methods studied showed major differences in outcome compared to the other.
Thus, surgical stabilization should be considered as the first line of treatment for immature patients with ACL tears. The existing literature suggests that transphyseal reconstruction can be safely done in this population if a few rules are considered, and there are physeal-sparing procedures that provide excellent results with less theoretical risk to the growth plate. Conservative or delayed surgical treatment, which carries an increased risk of secondary joint injury, should be reserved only for selected cases [62].
This change of perspective has not been fully agreed upon especially regarding surgical procedure with respect to physeal growth plates. Moksnes et al. [63] performed a recent literature review and found that there is no consensus on the management of anterior cruciate ligament injuries in skeletally immature, and that the methodological quality of published studies is questionable. The transphyseal reconstructions, physeal-sparing reconstructions, and nonoperative treatment algorithms that are advocated have little supportive data.
In the literature the standard bone age evaluation for pediatric ACL reconstructions is the one proposed by Tanner and Whitehouse; this system scores 20 indicators on hand and wrist radiograph, yielding total scores ranging from 0 to 100. Even though we were not able to use this scale in retrospect the probable estimate Tanner stage in our patients was III and IV. Example of MRI appearance can be seen in Figs. 3.6, 3.7, 3.8, and 3.9.
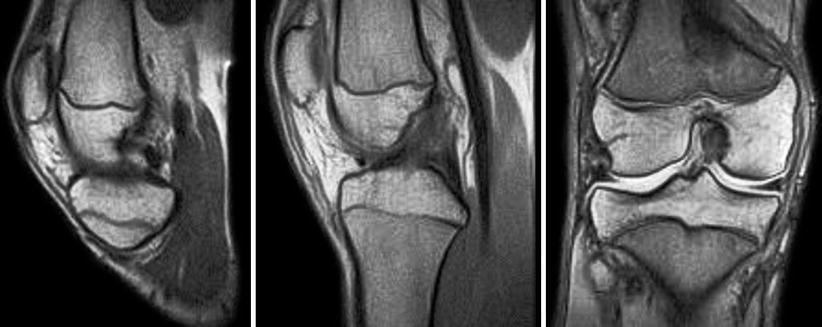
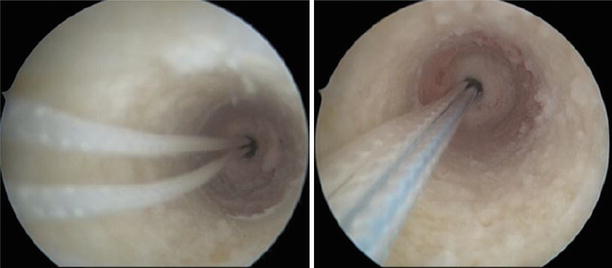
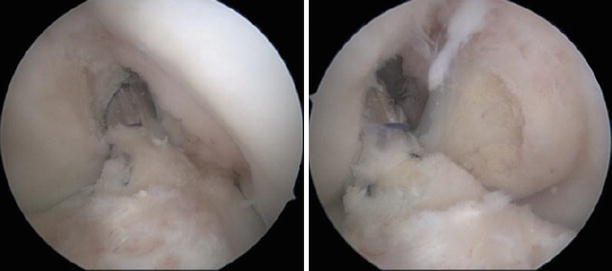
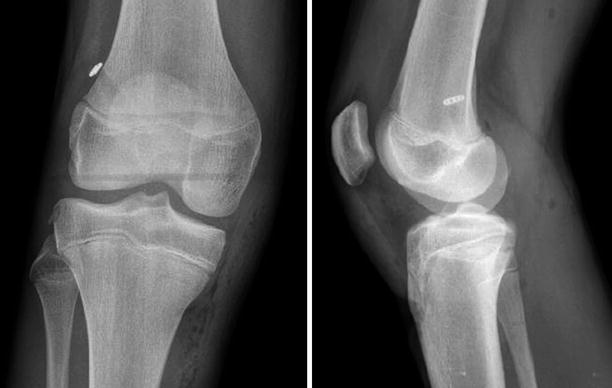

Fig. 3.6
RISE and T1 sagittal and T1 coronal views of 16 years old boy with open physis and torn ACL

Fig. 3.7
Femoral tunnel of two cases showing the arthroscopic view of the transphyseal ACL reconstruction [64]

Fig. 3.8
Lateral portal views of the case above of 16 years old boy with anatomic single bundle (transphyseal) ACL reconstruction using ipsilateral hamstrings (semitendinosus and gracilis) autograft (quadruple) [64]

Fig. 3.9
Show AP and lateral radiographs of a 16 years old boy with open physis and ACL reconstruction using transphyseal technique with hamstrings autograft, cortical femoral fixation and absorbable tibial interference screw [64]
Similar studies have found favorable results for autologus hamstrings grafts using transphyseal techniques [65–67]. Redler et al. [68] showed that transphyseal ACL reconstruction with autogenous quadrupled hamstring graft with metaphyseal fixation in skeletally immatures (14.2 years) yielded excellent functional outcomes in a high percentage of patients without perceived clinical growth disturbance at a mean of 43.4 months.
Drill hole placement during ACL reconstruction produces a zone of physeal injury. The overall volume of injury is relatively low, which reduces the risk of physeal arrest. With careful placement, the region of injury is central on the tibia, and the total volume of injury can be less than 5.0 % of the physeal volume. For the femur, the total volume can be less than 5.0 % as well. However, the region of injury is peripheral, which carries a higher risk of physeal arrest. For the tibia, drill holes that started more medial, distal, and with a steeper angle of inclination reduced the amount of physis and apophysis violated when compared with holes placed more lateral, proximal, and with a shallow angle of inclination [69–71].
Other authors [71] have used MRIs to identify physeal sparring directions of the femoral tunnel using transphyseal techniques and proposed drilling from the center of the ACL femoral footprint to the insertion of the popliteal tendon; this could result in a mean tunnel length of 27–30 mm, and it might allow the safe placement of a femoral tunnel at least 7 mm in diameter in patients 6–17 years old. The center of the ACL femoral footprint and the popliteal insertion are easily identifiable landmarks and will allow safe, reproducible, anatomic ACL reconstruction in the skeletally immature patient.
A retrospective case series of 933 knees with a mean age at the time of surgery of 15 years and an average follow-up from index surgery of 6.3 years evaluated the overall prevalence of arthrofibrosis and found it to be 8.3 %; 77 knees had at least one procedure to treat arthrofibrosis after ACL reconstruction. Arthrofibrosis was defined as a loss of 5° or more of extension or a loss of 15° or more flexion compared with the contralateral knee that required a follow-up procedure. Risk factors for arthrofibrosis were female sex, higher age (16–18 years odds ratio 3.51), patellar tendon autograft (odds ratio 1.7), and concomitant meniscal repair (odds ratio 2.08). Prior knee surgery and ACL reconstruction within 1 month of injury were not significantly associated with arthrofibrosis after ACL reconstruction [72].
Frosch et al. [73] identified a total of 55 articles reporting on 935 patients (median age 13 years) with a median follow-up of 40 months; their review found the weighted rate of leg-length differences or axis deviations was 1.8 % and that of reruptures was 4.8 %. Excellent or good function (International Knee Documentation Committee grade A or B) was achieved in 84.2 % of knees and Lysholm scores averaged 96.3. Transphyseal reconstruction was associated with a significantly lower risk of leg-length differences or axis deviations compared with physeal-sparing techniques (1.9 % versus 5.8 %; relative ratio 0.34) but had a higher risk of rerupture (4.2 % versus 1.4 %; relative ratio 2.91). Sutures did not result in any growth disturbances, with a weighted rerupture rate of 4.6 %. Fixation far from the joint line fared better than close fixation with regard to this endpoint (1.4 % versus 3.2 %; relative ratio 0.42). Bone-patellar tendon-bone grafts, which are also less likely to fail, were associated with higher risks of leg-length differences or axis deviations than were hamstrings (3.6 % versus 2.0 %; relative ratio 1.82). Meta-regression did not show a significant impact of the publication year on event rates.
Kaeding et al. [74] aimed to determine whether any anterior cruciate ligament reconstruction technique is clinically superior in skeletally immature patients having at least one of the following criteria: chronologic or bone age of less than 15 years in boys or less than 14 years in girls, Tanner stage I, II, or III and at least 10 cm of total growth after the reconstruction. Thirteen case series were included. Four studies used physeal-sparing techniques. Six studies used transphyseal techniques. Two studies used a combined technique, and a multicenter study reported results of both techniques. Within the physeal-sparing group, there were two studies that used an entirely extra-epiphyseal technique and two studies that used intra-epiphyseal techniques. Overall clinical outcomes were excellent, with growth complications being very rare in all of these series. The authors concluded that both physeal-sparing and transphyseal reconstructions can produce excellent clinical outcomes with a very low incidence of growth complications in Tanner stage II and III patients. Tanner stage I patients had excellent clinical results with physeal-sparing techniques (both extra- and intra-epiphyseal techniques). Not enough Tanner stage I patients underwent transphyseal techniques to support or discourage their use. This evidence supports considering the expansion of transphyseal reconstruction indications from Tanner stage IV patients to Tanner stage II and III patients.
3.4 Graft Healing and Stump Preservation
ACL (anterior cruciate ligament) reconstruction is a constantly evolving procedure. The ever-changing trends follow the common goal to restore preinjury anatomy and function as closely as possible [75]. With all the progress that has been made during the last years towards better understanding of the graft healing process and individually tailored surgery and rehabilitation, this target has not yet been fully achieved.
After reconstruction, the neoligament goes through a series of transformations that will lead to graft – tunnel integration and ligamentization of the intraarticular portion; this later process brings the tendinous structure closer to the original ligament. Amiel et al. proposed the term ‘ligamentization’ to describe the gradual transformation of the autologus B-PT-B graft into a neoligament that resembled the native ACL. The histological and biochemical metamorphosis occurred by 30 weeks and led to ligamentous cell morphology and increased concentration of type III collagen, not normally present in the patellar tendon as well as increased content of glycosaminoglycans [76].
The early phase is characterized by necrosis and hypocellularity. It lasts for the first month, during which the graft depends on synovial fluid nutrition [77]. After 6 weeks, revascularization occurs and the graft goes through intense cell proliferation. This intermediate period lasts until 3 months postoperatively. The final stage of structural remodeling continues over 6 months. In this later period, the graft begins to resemble the native ACL. This process was described for allografts and studied on animal models. During the early stages of revascularization and cell proliferation, allografts show significant delay compared to autografts. These differences start to fade by 1 year even though they continue to exhibit lower mechanical properties and anterior-posterior laxity [78].
It has been long suggested that preservation of the ACL stump and the Hoffa fat pad might be beneficial, especially for the early healing period [79]. However, while the grafts remain viable during this course, complete necrosis and cell replacement in humans does not follow the animal model [80]. Histologically the neoligaments resemble the native ACL but ultrastructural differences of collagen fibers distribution do persist [81]. One of the topics that are still debated is defining the importance of ACL remnants for the final functional outcome. Moreover, restoring proprioception of the ACL reconstructed knee has been hypothesized to be an integral part for successful outcome. The ACL has very poor healing capacity manly due to poor vascularization that is even further damaged after injury.
Denti et al. [82] and Barrack et al. [83] have found mechanoreceptors after ACL reconstruction with tendinous grafts and proposed that a reinnervation of the neoligament had occurred. Georgoulis et al. [84] using gold staining found that Ruffini and Pacini type mechanoreceptors existed in the subsynovial layer and near the femoral and tibial insertions of the ACL in remnant stumps connected to the PCL. Adachi et al. [85] observed a positive correlation between the number of proprioceptors and the accuracy of joint position sense. The authors even found mechanoreceptors in patients having a long interval between injury and the ACL reconstruction and concluded that surgeons should consider preserving ACL remnants to improve clinical outcome.
Gohil et al. [86], in a randomized control trial investigated whether limited debridement of the intercondylar notch and the residual stump of the ruptured ACL leads to earlier revascularization after ACL reconstruction using autologous four-strand hamstring tendon grafts as evaluated by postoperative MRI. Their results indicate that minimal removal of remnants leads to earlier revascularization within the mid-substance of the graft over midterm evaluation.
Lee et al. [87] presented results of the 16 subjects that had undergone ACL reconstruction with the remnant-preserving technique described in a previous article at a mean follow-up of 35.1 months. At the last examination, the patients were evaluated with the IKDC scale and HSS score as subjective tests; stress radiographs, Lachman test, and anterior drawer test by use of the KT-2000 arthrometer as objective tests and single-legged hop test, reproduction of passive positioning, threshold to detection of passive motion, and single-limb standing test as functional tests. On the basis of the extent of ACL remnant, patients were divided into more and less than 20 % respectively. A statistical comparison of the final results revealed that the difference was not significant in terms of mechanical stability, but a significant difference was detected in functional outcome and proprioception regarding the threshold to detection of passive motion at 30° and reproduction of passive positioning at 15° and 30°. In a subsequent study, [88] Ruffii (globular or ovoid corpuscle with thin capsule) and Golgi (fusiform corpuscle with thin capsule) type mechanoreceptors were found predominantly around insertion sites, located in the subsynovial layer and rarely in the interfascicular matrix in the normal (control) ACLs and more uniformly distributed in the remnant stumps collected from injured knees. Immunohistochemical staining method proved to be reliable and relevant in terms of specifically identifying the presence of mechanoreceptors in the normal and tibial remnant of the ruptured ACLs [89]. In the control group 40 % of slices contained mechanoreceptors. However, only one third of the remnant group showed viable mechanoreceptors (one or two per stump) and these were identified in 11 % of the total slices.
Dhillon et al. [85] examined ACL remnants by conventional light microscope. The monoclonal antibody against NFP stained the axons cylinders carrying proprioception. The monoclonal antibodies against S-100 stained free nerve endings and Schwann cells. H&E was used for detailed histological evaluation of synovial lining, sub synovial vascularity, intravascular morphologically normal mechanoreceptors, degenerative changes, and inflammation [90]. Papalia et al. [78] presented a literature review regarding the rationale for preserving the ACL remnants during reconstruction. Seven studies were included. The authors observed that ACL remnants might accelerate the vascularization and the ligamentization of the graft and contribute to faster graft innervation leading to a better proprioception. However, the current assessment methods do not lead to hard evidence that preservation of the remnant confers clinically relevant advantages over its excision.
Our tibial stump preservation technique is comparable to that described by Lee et al. [91] and Locherbach et al. [92] in the sense that ACL remnants are used as a biological sleeve for the graft. We also use ipsilateral quadruple hamstrings autograft and the tibial tunnel was performed inside and through the tibial ACL stump by careful sequential drilling that stopped before completely penetrating the ligamentous remnant.
One concern with tibial remnant preservation is the potential for intraoperative or future roof impingement. We found that accurate placement of tibial tunnel exit point, careful progression of pulling sutures through the middle of the remnant and sizing of the graft diameter prevents overstuffing the intercondylar notch. Nevertheless, occasionally we had to perform minimal adjustments of the ACL remnant after the graft was in final position to prevent progression towards future development of cyclop-like lesions due to stump preservation [93].
The ACL is, as are all key ligaments, important in defining proprioception and body spatial positioning using mechanoreceptors and neural endings. The role of this structures have only recently been credited to their full potential and importance in early recovery, prevention of reinjury and full return to pretrauma body kinematics. Whenever remnants are preserved, bony landmarks remain covered and potential for tunnel misplacement can arise. We consider that preservation of the tibial remnant only does not alter significantly the orientation (Figs. 3.10 and 3.11).
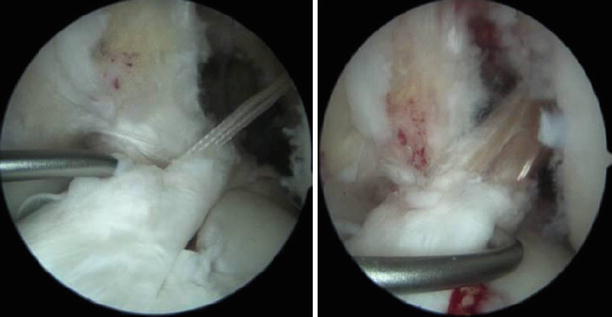
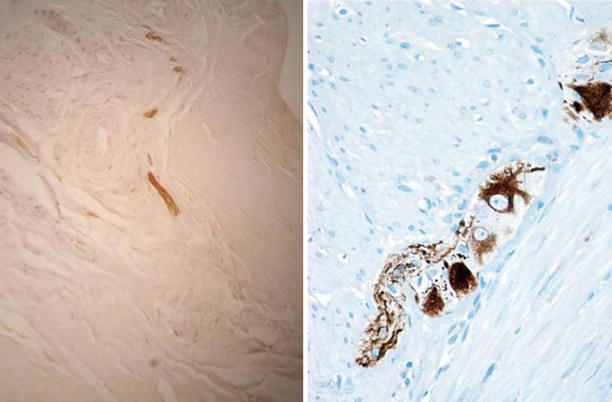

Fig. 3.10
Arthroscopic view of the exit point of the tibial tunnel with the remnant ACL stump preserved and showed with the probe; in the second image, the neoligament passing through the remaining tibial insertion and surrounded by the stump to promote healing whilst preventing impingement

Fig. 3.11
Focal free nerve terminals evidenced by immunohistochemical S-100 staining and mechanoreceptors from the tibial biopsy stained using anti-NF
3.5 Current Anterior Cruciate Ligament Reconstruction
Anterior cruciate ligament (ACL) lesions and their treatment are one of the most debated topics in orthopedics and knee surgery today. While the surgical indication still is a question to some, most orthopedic surgeons agree that reconstruction is the only viable option for a successful management of an ACL deficient knee. This being said, the debate continues as to which graft is most suited for a better outcome, which implants from the wide array that are available on the market have the best results and even which technique is the best.
3.5.1 The Transtibial Technique
The transtibial technique (TT) has been the first widely used arthroscopic reconstruction technique, with favorable outcomes reported in the late ’80. In the late ’90, the works of Professor Freddie Fu from Pittsburg and others have raised concern regarding the graft placement and function using this surgical procedure. In addition, the clock dial referencing was considered an inappropriate single plane description of a three dimensional position. More research into the biomechanics of the ACL saw the advent of the double bundle technique (DB).
The over-the-top position of the graft on the femur was anterior and medial to the native ACL insertion [9, 17]. In knees with hyperextension and a vertical notch roof, the TT technique required notchplasty and a more posteriorly placed tibial tunnel to prevent impingement [94]. This led to extremely vertical and nonphysiological grafts that were found to have less resistance to anterior translation and especially less rotational stability compared to double bundle anatomic reconstruction [95]. The current switch to anatomic reconstruction has greatly limited the need for notchplasty, mainly to osteophyte removal from the lateral condyle border (Fig. 3.12). Experimental studies have repeatedly showed that the femoral attachment of the native ACL is on the lateral femoral condyle and never on the notch roof. A correctly positioned tibial tunnel will never allow for a correct placement of the femoral aperture in the native insertion [96]. Further research provided additional data to support femoral tunnel drilling through the antero-medial portal. When compared to the TT technique, it offered better function and closer obliquity to the native contralateral ACL [97, 98]. With regard to the tibial tunnel aperture, the results are somewhat inconclusive between the two techniques. It has been shown that trans tibial technique places the tibial tunnel in the same position as the trans antero-medial portal or more posterior in order to achieve better placement of the femoral tunnel [97–99].
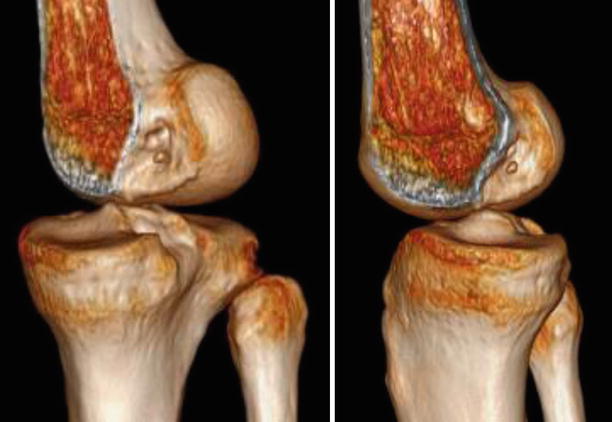

Fig. 3.12
A patient with previous single bundle ACL reconstruction using the transtibial technique (TT) and ipsilateral patellar tendon autograft. 3D CT (three dimensional computed tomography) reconstructions shows the high, non anatomic placement of the femoral tunnel on the roof of the notch
It is now universally accepted that ACL reconstruction via transtibial technique fails to accurately position femoral and tibial tunnels within the natural insertion site [100]. In addition, freedom of femoral drilling through the AM portal will also allow for a more anterior placement of the tibial tunnel [99]. This in turn will lead to a more oblique and natural graft placement with improved restoration of anatomy and stability with ACL reconstruction compared with conventional transtibial drilling techniques [101].
The introduction of the anatomic ACL reconstruction technique changed the way surgeons drill the femoral tunnel, by using an antero-medial portal instead of the traditional tibial tunnel. This subsequently changed the positioning of the tunnels and the resulting obliquity of the graft. One of the first materials that evaluated neoligament obliquity in ACL reconstructed patients found a continuous and homogeneous graft similar to the native ACL, but with a more vertical position that does not recreate the normal sagittal obliquity. Nevertheless, these more vertical grafts were still found to control anterior posterior knee displacement [102].
Ahn et al. compared the antero-medial and TT techniques and found a significant vertical angle in the coronal and sagittal plane for the TT reconstructions compared with the native ACL. In addition, the more horizontally the angle of the tibial tunnel can be the closer will be the result compared to the native ACL [103] When the tibial remnant stump was preserved, magnetic resonance imaging showed significantly larger grafts with progressive remodeling and no increase in the incidence of cyclops lesions [104]. MRI proves to be the most useful imaging method in determining outcome after ACL reconstruction. It gives reliable information on graft healing, integrity, length, position, inclination angle, obliquity, impingement syndromes and potential associated lesions. It may even identify ACL impingement against PCL (posterior cruciate ligament) with the knee extended, which cannot be detected by conventional arthroscopy [105].
Three dimensional (3D) volume rendered (VRT) computed tomography (CT) is a useful tool for planning accurate femoral tunnel positioning when aiming for anatomic ACL reconstruction. The direct insertion of the ACL is located in the depression between the resident’s ridge and the articular cartilage margin on the lateral femoral condyle [106]. 3D CT reconstructed volumes (VRT) are reliable and can be used to assess the tunnel position regarding stability and outcome [107]. Although not as widely used, 3D MR imaging were proved accurate and can also be used for preoperative templating in anatomic ACL reconstruction (Figs. 3.13 and 3.14) [108].
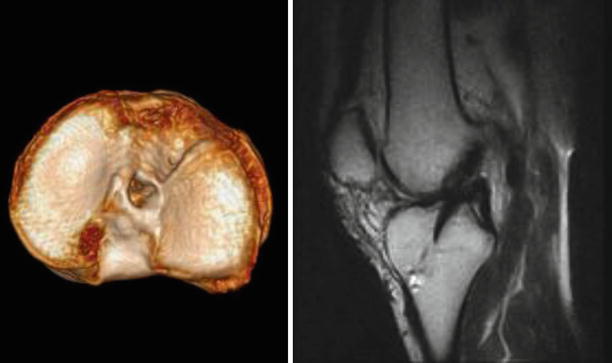
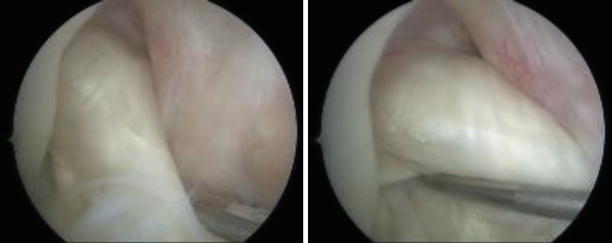

Fig. 3.13
The same case, 3D CT volume rendering showing the non-anatomic, posterior tibial tunnel aperture. The result is a vertical although homogenic hypointense aspect of the neoligament on the sagittal T2 MRI (right)

Fig. 3.14
Arthroscopic views of the same case through the anterolateral portal showing a healed and integrated vertical graft; the arthroscopic evaluation was performed in order to perform a partial medial meniscectomy resulting from a recent noncontact sporting injury
3.5.2 The Anatomic Single Bundle Reconstruction
The anatomic single bundle technique appeared initially as a therapeutic alternative in cases where anatomic DB technique could not be performed (small knees, narrow notch) and later gained increased use by surgeons due to easier technique, shorter operating room time and comparable results to the DB technique. The anatomic single bundle (SB) reconstruction technique has been found to more accurately reproduce the femoral footprint and the orientation of the graft. By comparison, with the classical TT technique the tibial tunnel placement resulted in a more vertical graft than native ACL. It has thus been underlined the importance of thorough knowledge of the anatomical landmarks of the ACL [109]. Nonetheless, in many cases normal graft obliquity is not restored with either technique. This may be caused by the single bundle technique itself [110].
The senior authors have been performing ACL reconstructions for more than a decade, with almost 1,000 treated cases each. In the beginning, we used the TT single bundle technique with autologous ipsilateral bone-patellar tendon-bone (B-PT-B) graft fixed with 2 interference screws. Presently, our most versatile and recommended procedure is the anatomic SB reconstruction with autologous ipsilateral quadrupled hamstring graft, cortical button femoral and interference screw tibial fixations. Nonetheless, many types of grafts and fixations can be used equally successful. This technique was applied to both professional and amateur athletes with excellent functional outcomes. A knee MRI is performed on a regular basis to investigate for possible associated ruptures of the menisci, lesions of the cartilage or lesions of the collateral ligaments even though clinical tests (Lachmann, anterior drawer test) are sufficient for the diagnosis of the ACL rupture (Fig. 3.15).
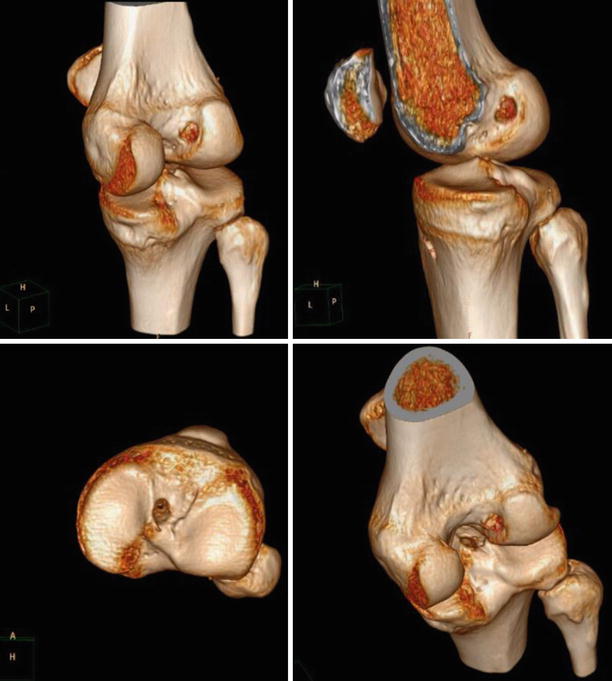

Fig. 3.15
By contrast, the 3D reconstructions of an anatomic single bundle reconstruction shows both tunnel apertures to be centered and sized to the native insertions
3.5.2.1 Surgical Technique
A clinical exam can be repeated after the patient is relaxed from the anesthesia. We do not perform routine exploratory arthroscopy if the preoperative evaluation is conclusive and proceed directly to graft harvesting (see Sect. 3.7). The arthroscopic exploration is performed in a standard fashion (see Sect. 1.2.4). We routinely only use two portals: a slightly higher and centrally placed antero-lateral and a lower and deeper antero-medial, to improve tibial aperture visualization and tunnel direction. Any associated lesions are addressed first; the remnants are parsimoniously debrided and the tunnels prepared while the graft is being handled on the back table. Stable longitudinal meniscal tears are left in situ, large, recent bucket handle tears are sutured and degenerative complex tears are excised. The target is to preserve as much meniscal tissue as possible (see Sect. 2.2) but the most commonly performed procedure in our patients is partial meniscectomy. For cartilage defects we usually perform microfractures (see Sect. 7.1).
What sets this technique apart from the classic single bundle ACL reconstruction technique is that the drilling of the femoral tunnel is done only after clear identification of the anatomical landmarks of the ruptured ACL. Initially we look for the femoral origin and we proceed with careful shaving to help delineate the borders of the ACL. In cases where the proximal remnant in no longer visible we debride the soft tissues until the bone is exposed. If visible, the intercondylar ridge is the superior limit of the ACL footprint with the knee in over 90° of flexion. For a better view of the notch we recommend moving the arthroscope from the antero-lateral to the antero-medial portal. The old “clock” theory that recommends the placement of the tunnel should be disregarded. One hundred and ten degrees flexion of the knee and an inferiorly placed medial portal are essential for the desired femoral tunnel aperture. The entry point should be the limit between the footprints of the AM and PL bundles, but not higher than the intercondylar ridge. The femoral ACL footprint is centered 1.7 mm proximal to the bifurcate ridge and 6.1 mm posterior to the lateral intercondylar ridge [111].
A mark can be made with the Steadman awl (used for microfractures) or with the guide pin freely or using the appropriately sized posterior wall guide (according the size of the graft). The position should always be checked from the central or medial portals. A 4 × 4 imaginary grid or intraoperative radioscopy can also be used but we do not routinely use them (Fig. 3.16). The appropriate placement of the femoral tunnel is a key point for a favorable outcome, and the leading cause of failure in cases that are revised (see Sect. 4.3).
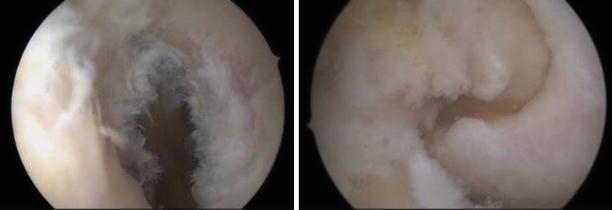

Fig. 3.16
Femoral footprint gently debrided, viewed from the antero-lateral portal with the left knee in high flexion. The only current indication for notchplasty is the presence of large osteophytes on the medial side of the lateral condyle
Once the position is correct, a graded pin is used to measure the distance to the lateral cortex and decide tunnel length. The use of a 9 mm drill at a transverse angle of 40° with the knee in 102° of flexion was shown to produce a femoral tunnel aperture area that best matched the native ACL footprint [112]. A cadaveric study found that when drilling femoral tunnels through the far antero-medial portal both high and low knee flexion angles could have the potential risks of damage to articular cartilage or the common peroneal nerve [113].
The femoral guide pin is entering through the antero-medial portal and exiting the lateral condyle in a correct direction (Fig. 3.17); progressive reaming to the desired size (usually 8) is achieved with special drills that have a thin rounded body and a short reaming tip. This shape is used to protect the medial condyle cartilage (Fig. 3.18). We recommend sizing the femoral reaming in 0.5 mm increments, especially when cortical button fixation is used. A tight fit of the soft tissue graft in the tunnel is highly desired both in diameter and at the end. Variable loop systems or the smallest loop that will ‘flip’ should be used. The leading sutures are passed through the loop at the end of the guide pin (Figs. 3.19 and 3.20).
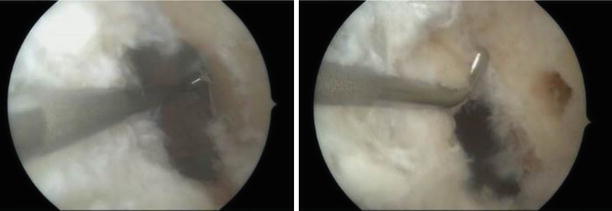
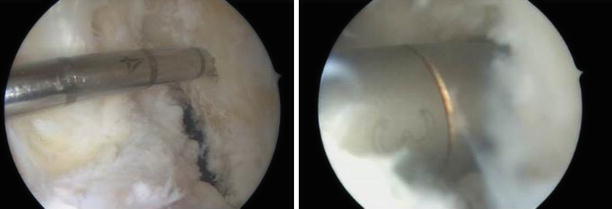
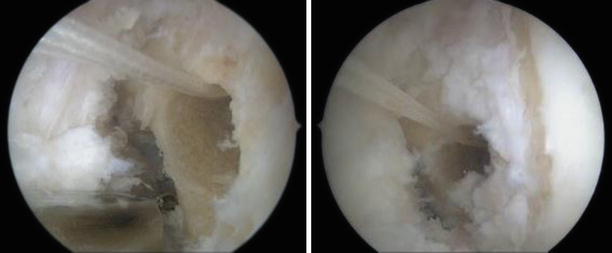

Fig. 3.17
The posterior cortex is referenced and shown in relation to the center of the femoral tunnel (antero-lateral portal view of the left knee)

Fig. 3.18
A graded 4 mm pin is used to measure the distance to the lateral cortex (34 mm) and decide tunnel length (25) and loop size (20); progressive drilling to the desired size (usually 8) (antero-lateral portal view of the left knee)

Fig. 3.19
Extra articular view of the left knee flexed to 110°; the patient is supine on a full table and the sterile draping is for general lower extremity use, without a fluid pouch. The arthroscope is through the antero-lateral portal and the femoral guide pin is entering through the antero-medial portal and exiting the lateral condyle in a correct direction

Fig. 3.20
Antero-lateral and antero-medial portal views of the left knee with the femoral aperture in the correct position and the leading sutures running trough
The best reference for the tibial tunnel aperture is the remnant ACL of the native ACL (see Sect. 3.1.2). The guide pin should exit in line with the native fibers, in the center of the stump (Fig. 3.21). If this landmark is not clear, as in old ruptures, the tibial insertion should be centered 7.5 mm medial to the anterior horn of the lateral meniscus, 13 mm anterior to the retro-eminence and 10.5 mm posterior to the ACL ridge [111]. The guide is set to 50–55°. The entry point is placed near the anterior border of the superficial MCL insertion with care not to damage the fibers during drilling. We use the same incision used to harvest the hamstrings or a small 1 cm separate incision if a different graft is used. The tibial tunnel should exit as far anterior and medial to prevent impingement in full extension. The tibial stump should also be trimmed to prevent anterior impingement due to cyclops formation. The more oblique the direction of the tibial tunnel is, the larger the intraarticular aperture becomes and the transition of the neoligament smoother.
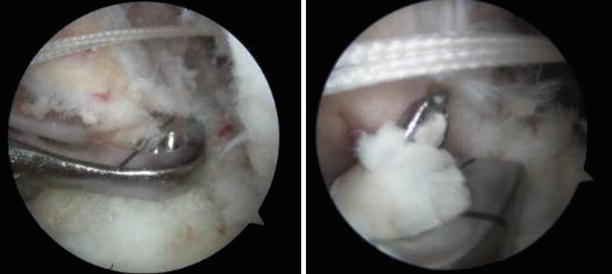

Fig. 3.21
The tibial guide pin is in the middle of the remnant stump (antero-lateral portal view of the left knee)
However, drills larger than 9 mm angled at 45° produce larger tibial footprints than the native ACL and will likely damage the structures surrounding the aperture [114]. We therefore progressively drill the tibial tunnel to the measured size to prevent proximal aperture irregularities. Furthermore, we also use the full tibia drills as opposed to the femoral designs since this also can affect the final shape of the tunnel [115]. The distal aperture is then debrided from fascial soft tissue and the proximal shaved of excessive residual stump to facilitate graft passage. The minimum recommended graft length in the bone tunnels is 15 mm. Several studies failed to obtain improved fixation of soft tissue grafts with higher lengths in animal models [116, 117].
Once the femoral end is stabilized, the knee is put into several flexion-extension motions for better adjustment of the graft tension in the tibial tunnel. With the knee in approximately 30° of flexion and 8 kg force of pulling on the ligament the tibial fixation is completed. The proximal tibia should be pushed backward to counteract the anterior pull of the help during tensioning. There is no clear data on the amount of tension required as well as the reproducibility in real conditions [118]. Applying reduced pulling tension during tibial fixation might leave the ACL graft slack. However, increased tension appears to impede the healing during the ligamentization process and favor the development of stiffness in the immediate postoperative period. Histological analysis of grafts implanted under higher tension forces showed more necrotic areas which led to increased ligamentous laxity compared to grafts with minimal initial tension. Especially tendon grafts, such as the semitendinosus and gracilis have viscoelastic properties and can increase the length by pretensioning (Figs. 3.22, 3.23, and 3.24) (see Sect. 3.7.1).
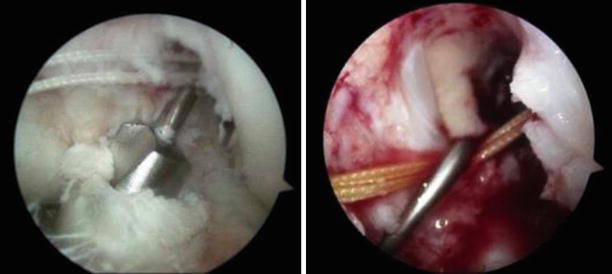

Fig. 3.22
Sequential drilling of the tibial tunnel (antero-lateral portal view of the left knee). “Hooking” the passing suture with the probe to pass it through the tibial tunnel (antero-lateral portal view of the left knee)

Fig. 3.23
The graft is pulled through the tunnels and the tension is maintained by pulling on the leading sutures; extra articular view of the left knee with the patient supine on the table with the leg segments removed and the feet hanging

Fig. 3.24
The graft in the final position (antero-lateral and antero-medial portal views of the left knee); the oblique anatomic placement leads to a triangle between the reconstructed ACL and the native PCL
We no longer use postoperative drains after primary ACL reconstructions, both intraarticular and at the hamstrings donor site. This shift in management was applied several years ago and did not lead to increased rated of effusions, hematomas or infections. All our patients receive pharmaceutical thromboprophylaxis by single subcutaneous injections of low molecular weight heparins up to 1 week. A compressive long leg stockinette is also worn for a minimum of 1 week. Immediate partial weight-bearing is permitted with the aid of 2 crutches for the first 3 weeks. We do not routinely immobilize primary ACL reconstructions but do use orthosis to limit range of motion when concomitant meniscal sutures are performed (see Sect. 2.3.1).
3.5.3 The Double Bundle Technique
The femoral and tibial insertion sites of the native ACL were extensively studied for landmarks, shape and normal variation (see Sect. 3.1.2). This was translated technically in the advent of “anatomic” techniques. The first to emerge was the anatomic double bundle (DB) technique that aimed not only to replicate the number of anatomical bundles of the ACL but also their insertion sites in order to restore knee kinematics to preinjury level [119]. There are several variations in the technique for DB reconstruction. Two standard portals are most commonly used, although some surgeons use a third central or accessory anteromedial to aid visualization. Transtibial DB is possible but the femoral aperture of the PL bundle is very unlikely to be accurately positioned through this approach [120]. A four tunnel approach is therefore recommended and used by the majority of surgeons. The patient is positioned in the same manner as for SB reconstruction. After a brief examination of instability under anesthesia, a standard exploration of the joint is performed (see Sect. 1.2.4).
By far the best graft choices for DB reconstruction are double looped hamstrings or tendon allograft with lateral cortical button fixation. Ideally, the grafts should be larger than 6 mm for the AM and 5 mm for the PL bundle respectively. We harvest the semitendinosus and gracillis tendons through a 3 cm oblique incision centered over their ‘pes anserinus insertion’. The semiT is used for the AM bundle which is thicker and longer and the gracilis for the PL (see Sect. 3.1.2).
While the grafts are prepared on the backtable, any associated lesions are addressed. We always try to preserve as much as possible from the menisci. Although we most commonly treat meniscal ruptures by partial meniscectomy, we try to perform limited excisions and whenever possible suture. Outside in techniques are cheaper but require more effort (see Sect. 2.3.1). Small, stable longitudinal ruptures, especially when located on the posterior horn of the lateral meniscus can be successfully left in situ [121]. The ACL remnants and especially the tibial are trimmed just enough to allow for footprint identification and prevent impingement. The medial side of the lateral condyle is gently debrided of soft tissues with a mechanical or radiofrequency shaver.
With the knee flexed at 110–120° the location of the AM and PL bundles are judged and their centers marked with an awl or a small pin (see Sect. 3.5.2.1). If only two portals are used, the position should also be assessed by switching the camera to the anteromedial portal. The AM bundle is placed higher and deeper in the footprint (see Sect. 3.1.2). The tunnel is reamed to the appropriate size and the corresponding guide is placed into the tunnel and rotated so that the PL is located positioned lower and more shallow. By using the guide seen in Fig. 3.25 the two femoral tunnels diverge at 15° and are separated by a 4 mm bonebridge. This is sometimes to wide and will force the tunnels to be located outside the anatomic landmarks. Smaller offset guides are currently available on the market. The tunnel is drilled through the lateral cortex with a 4 or 4.5 guide pin, depending on the fixation system. The AM tunnel is longer and wider and exits more proximal through good cortex. The PL tunnel is shorter and exits more lateral. Care should be taken when reaming because the lateral cortical bone is thin and can easily be reamed through.

Fig. 3.25
A comparison of the SB and DB tibial and femoral guides, showing how the AM tunnel guides the distance to the PL apertures
The tibial insertion is wider than for SB. The AM bundle aiming device is positioned just medial to the tuberosity, in a more anterior position than for SB and is passed at 55° to the horizontal. It is enlarged to the corresponding size and the appropriate PL drill guide is inserted through the AM tunnel and advanced until it becomes visible on the articular surface. The barrel has a mark on its head that indicates the direction of the PL guide pin. By rotating the guide’s handle this line will point toward the native PL bundle attachment (see Sect. 3.1.2). Using this guide, the PL aperture is placed 9 mm posteriorly and laterally to the center of the AM bundle tunnel (Fig. 3.26). The tunnel is then reamed to the graft size. Two different leading sutures are pulled through each femoral tunnel and brought distal from the articular side through the tibial tunnels using a grasp.
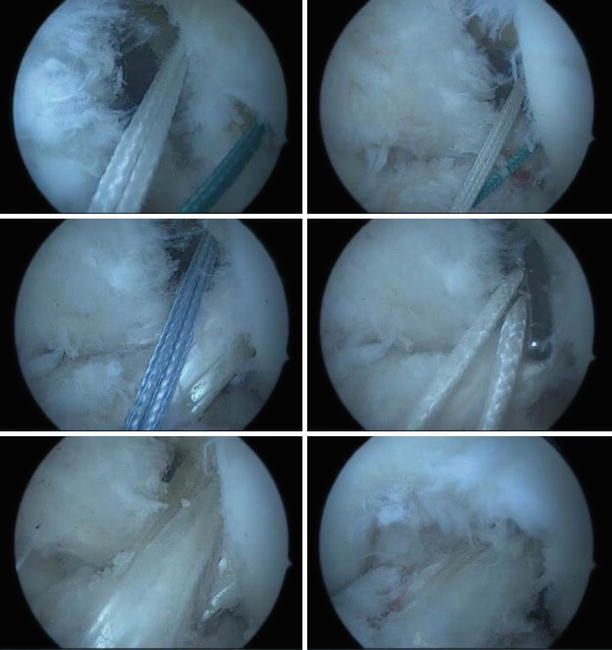

Fig. 3.26
Anterolateral portal views of the left knee. The femoral and tibial apertures of the two bundles are visible. A with leading suture is passes through the AM bundle and a green one through the PL respectively. The PL bundle is pulled through first, followed by the AM. The final aspect with the knee in flexion and extension
The PL bundle graft is pulled first and the AM second, through their corresponding tibial and then femoral tunnels, and the cortical buttons are secured. The small size of the femoral tunnels and their proximity on the lateral condyle makes interference screw fixation more difficult. The knee is then extended and flexed whilst individually manually tensioning the grafts. This assures a comfortable fit of the grafts in the tunnels. The AM bundle should be relatively isometric whereas the PL bundle should exhibit a perceptible amount of variation during the range of motion. The AM bundle is secured first at 50° of flexion. The angle for the PL bundle depends on the length change throughout the knee flexion. If this is less than 3 mm, the bundle should be fixed in 20° of flexion. The more variation is seen, the closer to full extension the fixation is performed. The tibial fixation is made using absorbable interference screws, one size larger than the tunnel diameter. The tension applied to the grafts should be between 5 and 8 kg of force. When in doubt, the tension can be measured using the coil dynamometer from the graft preparation bench. The remainder of the grafts can be cut flush to the cortex or the pulling sutures tight one to another over the bone bridge between the tunnels.
3.5.4 The Individualized ACL Reconstruction
The management of a ruptured ACL can differ depending on the patient age, past and future level of activity and the type of injury. For the elderly, sedentary patient conservative treatment and physiotherapy leads to a satisfactory outcome [122]. On the other hand, ACL deficient knees exhibit rotational instability even during activities of daily living. Gait analysis found these patients rotated their tibia internally during the initial swing phase. This abnormal movement might lead to articular degeneration and is attenuated after ACL reconstruction [123].
For the high demand patients such as athletes that wish to return to their previous level of exercise the primary goal is achieving a stable knee, avoiding further damage to the internal structures of the knee (menisci) and early start of rehabilitation and return to sports. Professional athletes are also one of the groups most exposed to the accidental rupture of the ACL. This injury is of the outmost importance to them because the ACL is the primary stabilizer of the knee. Injuries to this ligament can seriously alter an athlete’s physical activity level or even end their sporting career. The focus on restoring the native ACL landmarks has led to the popularization of the anatomic double-bundle technique. Recent data however has somewhat failed to show definitive superior outcomes with the double bundle approach. Nonetheless, the anatomic reconstructions, weather single or double bundle have proved superior to the transtibial technique.
The team led by Professor Freddie Fu from Pittsburgh, USA, have devised a flowchart individually tailor ACL surgery. The authors define anatomic reconstructions as the ‘functional restoration of the ACL to its native dimensions, fiber orientation, and insertion sites’ [73]. This guide requires large footprints to be restored by double bundle procedure in order to provide a closer resemblance to the native architecture. Nevertheless, for smaller knees and footprints the double-bundle is no longer considered superior [124]. An award winning paper based on a comparative study between techniques found that anatomic SB reconstruction resulted in better antero-posterior and rotational stability than classic SB reconstruction but the results of the anatomic DB were superior to both. However, the difference was significant only between anatomic DB and conventional SB, but in this particular study, the authors did not account for the individual footprint variability [125].
Hence, the most commonly listed indications for DB ACL reconstructions according to Musahl et al. are [126]:
large insertion sites (18 mm tibial ACL anteroposterior diameter),
wide notch width (12 mm),
high-grade pivot shift, and
revision ACL reconstruction
Established contraindications for double-bundle ACL reconstruction are:
multi-ligament injuries,
open physes,
degenerative OA,
small insertion sites (12 mm tibial anterior–posterior diameter), and
small notch width (12 mm)
To perform individualized reconstruction, the footprints of the insertion are measured (Fig. 3.27). These can also be sized on the preoperative MRI so that adequate graft choices can be made (Fig. 3.28)
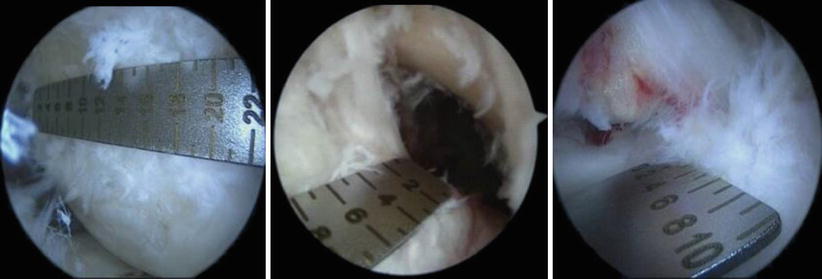
).

Fig. 3.27
Anteromedial portal measurement of the femoral insertion length on the lateral condyle using a ruler. Anterolateral views of the tibial footprint, measured for length and width. Based on these measurements, an anatomic SB reconstruction was chosen

Fig. 3.28
Anatomic SB reconstruction. The femoral and tibial footprints are identified: posterior to the lateral condylar ridge with the tunnel drilled through the anteromedial bundle; the tibial insertion, medial to the anterior horn of the lateral meniscus, anterior to the PCL, in the center of the remnant stump. The final graft in place, views from the anterolateral portals (Gruia)
The individualized approach takes into account each ACL footprint size [127]. Depending on the tibial insertion length, the recommended reconstruction technique is either SB, DB or both:
<14 mm with a notch width <12 mm – SB
14–18 mm with a notch width >12 mm – SB or DB
>18 mm – DB
3.5.5 Single Bundle Augmentation
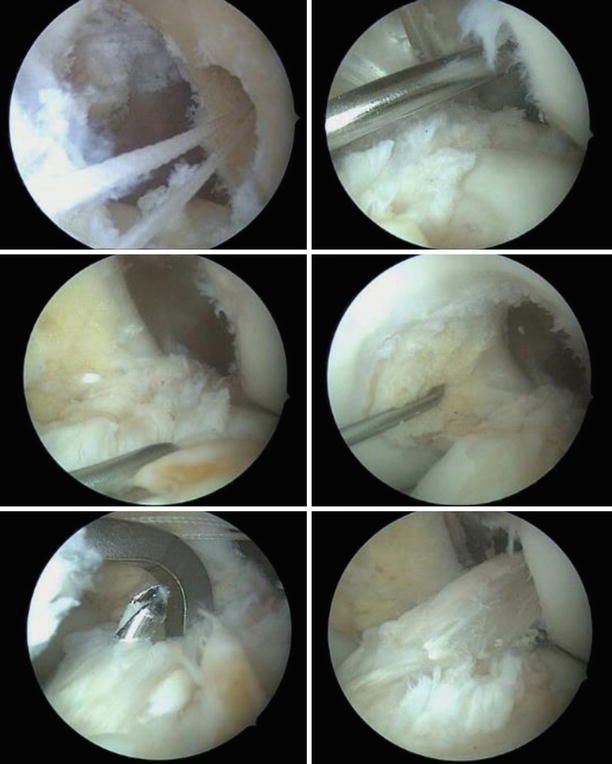
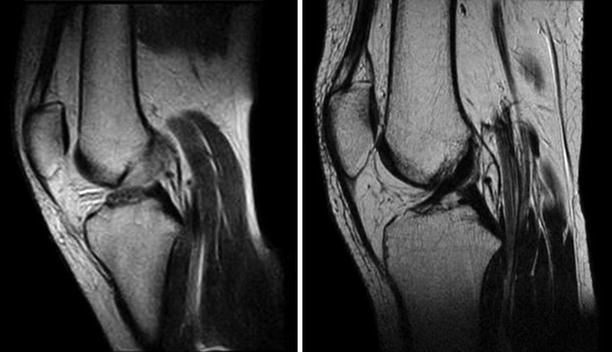
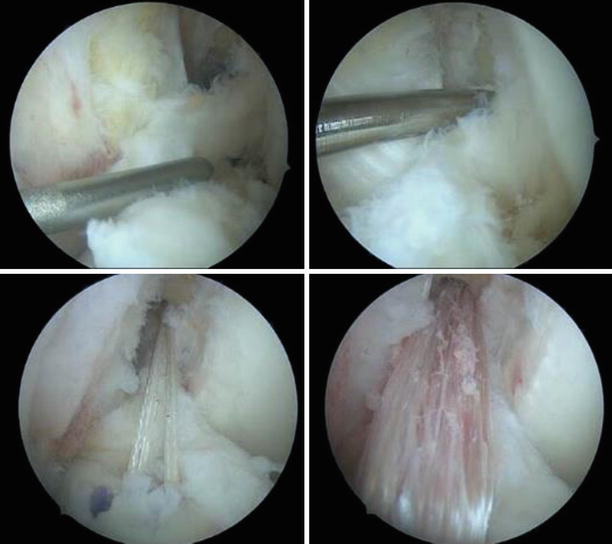
If clinically important instability is absent, partial tears usually have favorable outcomes with conservative treatment (Fig. 3.29). Approximately one-quarter will develop a positive pivot shift and might require reconstruction. When arthroscopic confirmation of the partial tear is obtained, selective bundle reconstruction can be successfully performed, although it can be challenging with a torn PL and preserved AM (Fig. 3.30) [128]
].

Fig. 3.29
Partial tear of the ACL that healed with conservative treatment as seen on repeat MRI after 2 years

Fig. 3.30
Anterolateral portal views of the left knee. The torn AM bundle was removed and the PL tautness is checked by pulling with the probe. The location of the AM insertion is identified, deeper and higher than the PL with the knee flexed. The guide wire is passed and the tunnel reamed to size 6. The AM graft (doubled semiT) is pulled through, seen from the anteromedial and anterolateral portals
3.5.6 Potential Pitfalls
Camera and hardware malfunction, incomplete instrument sets and missing sizes for the fixation system – always have alternatives for backup.
Undiagnosed associated instabilities: PLC, PCL, MCL – start with examination under anesthesia.
The Tourniquet is too distal or the femoral tunnel is too vertical and the guide wire will pierce the band – check and reposition.
Iatrogenic medial condyle reaming with the femoral drill – more knee flexion or the anteromedial portal is too deep (medial) (Fig. 3.31).
The femoral guide pin direction is too horizontal, resulting in a short tunnel with improper external cortical thickness for button fixation – more knee flexion is required or alternative fixation with a screw.
The femoral tunnel aperture is too posterior and the direction too oblique from shallow to deep, resulting in posterior cortex blow-out: a serious complication, very difficult to correct – cortical button fixation and limited postoperative range of motion; use the offset guide to place the pin entrypoint.
The femoral drilling is blind because the Hoffa pad obstructs the view from the lateral portal during flexion – shave the tissue, decrease the flexion angle, use an alternative central or accessory antero-medial portal.
The tibial tunnel is drilled with the aperture misplaced – a small adjustment can be made between the first and last sequential reaming by changing the position of the guide wire (Fig. 3.32).
The graft is dropped on the floor before fixation – be careful when handling, keep in disinfectant solution and lavage abundantly with saline; monitor for early signs of infection.
The graft is too lose after fixation – unscrew the tibial side and stabilize under appropriate tension.
A short graft is found in the tibial tunnel after femoral fixation or the traction sutures are cut during tibial screw fixation with the short graft inside the tunnel – remove femoral fixation and check tunnel lengths when using short grafts.
Surgical site infection, usually at the tibial tunnel – debride and even use small vacuum; check that the graft is cut flush to the cortex and the fixation hardware is not protruding; lavage the debris before careful layered closure.
Large and painful early postoperative effusion – needle aspiration, rest, ice and compressive bandage; suspicion of septic arthritis require early (and possibly repeated) arthroscopic lavage and antibiotics even before positive cultures are obtained; if the graft is not necrotic it can be left in place.
Important pain and edema of the lower limb – exclude deep vein thrombosis (Doppler ultrasound), use compressive stockinettes, rest and nonsteroidal anti-inflammatory medication, adjust the rehabilitation regimen (NSAIDs).
Extension deficit – allow early range of motion exercises, confirm patient adherence to an adequate rehabilitation program, exclude arthrofibrosis, cyclop lesion and tunnel malposition, check and document (arthroscopic pictures) the absence of roof impingement at the end of the surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








