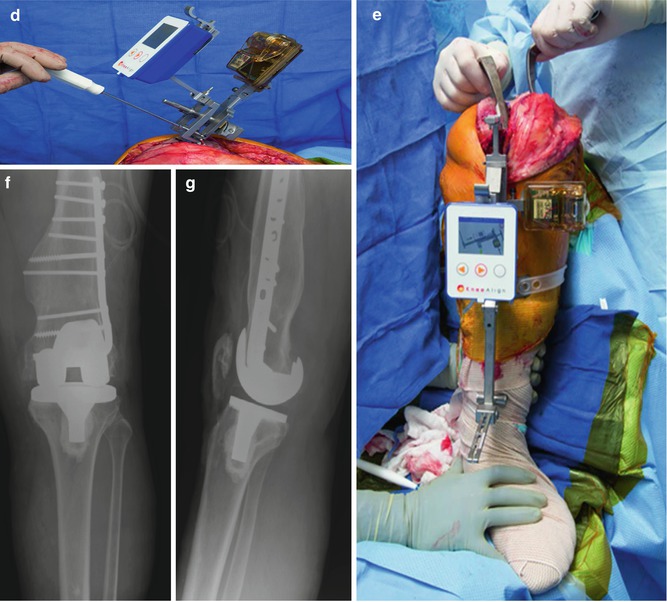
Fig. 11.1
Anteroposterior (AP) (a), lateral (b), and standing long-leg (c) preoperative radiographs of a 50-year-old gentleman with advanced tricompartmental post-traumatic arthritis, significant deformity, and in situ hardware. The complex primary TKA was completed with an accelerometer-based, portable navigation device for the distal femur (d) and proximal tibia (e) resections. Without removing the hardware or cannulating the femur, the postoperative coronal (f) and sagittal (g) alignments were acceptable
11.2.1 Alignment
In the coronal plane, substantial evidence supports superior alignment with navigated TKAs [9, 12–14]. In a large meta-analysis, Hetaimish et al. demonstrated improved mechanical alignment [12]. This is consistent with other meta-analyses [9, 13, 14]. Some cohort studies have also supported improved femoral coronal alignment [15–17], especially for patients with valgus deformities [18]. However, others have revealed no difference [19]. The majority of studies have failed to show a significant difference in tibial component alignment [15, 19], but conflicting reports exist as well [17]. Other meta-analyses have shown fewer outliers in the coronal and sagittal planes for both the femoral and tibial components [9, 12].
One recent study demonstrated a sevenfold increased risk of postoperative pain with femoral rotational malalignment, whereas coronal and sagittal alignments were not significant predictors of pain [7]. Some investigations have shown no difference in rotational alignment outliers for the tibial component [20], while the ability of navigation to improve rotational alignment of the femoral component remains controversial [12, 20, 21].
11.2.2 Patient Outcomes
Despite some reporting improved alignment with navigated TKAs, long-term studies show equivocal results with regard to patient outcomes [17, 19, 21–26]. In a prospective study of 54 patients, Knee Society scores (KSS) were similar between the navigated and conventional groups at 2.5 years [22]. Longer-term studies have reported similar KSS at 5 years [17, 23, 24, 26] and 10.8 years [19]. However, a randomized controlled trial (RCT) of 97 conventional and 98 navigated total knees demonstrated improved KSS, with significantly better improvement in pain compared to conventional TKAs [25]. A recent meta-analysis of over 7,151 TKAs also suggested higher KSS and WOMAC scores with computer-assisted navigation [21]. Conflicting evidence may result from inconsistent methodologies and varied component design.
11.2.3 Survivorship
Data is lacking to make any definitive conclusions about survivorship. A prospective study of 520 bilateral TKAs randomized to CAS vs. conventional techniques found similar survivorship at a mean of 11 years [19]. No other series has followed navigation for 15 or more years postoperatively. In comparison, survivorship of contemporary TKAs is over 85 % at 20 years [27, 28]. With few events at 10 years, longer-term studies are necessary.
11.2.4 Extramedullary Referencing
Using the femoral canal to assess alignment is difficult in cases with excessive angulation of the femur or in situ hardware. In one retrospective series of patients with prior femoral fractures, over 50 % of conventional TKAs did not achieve alignment [29]. Corrective osteotomies combined with TKAs (either staged or simultaneous) or extramedullary referencing can be used in such cases. Wang et al. popularized simultaneous osteotomy and TKA for extra-articular deformities [30]. However, simultaneous techniques show higher rates of nonunion, arthrofibrosis, infection, and pulmonary embolism [31]. On the contrary, many have reported good results using computer-assisted navigation, with 90 % achieving a neutral mechanical axis (±3°) [32–38].
11.2.5 Costs and Operative Efficiency
Widespread use of navigation is limited by costs. Equipment purchasing, training requirements, prolonged operative times, and maintenance fees substantially add to the total [20]. An increase of 8–63 min has been reported when utilizing navigation [10, 39–42]. One systematic review estimated an additional 20 min of operative time for navigation [43]. However, some authors argue that using adjustable cutting blocks significantly reduces operative time [44, 45].
Novak et al. advocated widespread use of navigation if it was within $629 the cost of conventional TKAs [46]. However, their analysis relied on improved alignment to reduce the need for revision arthroplasty. Two long-term series have recently questioned this relationship, showing similar survivorship between mechanically aligned and malaligned total knees [47, 48]. If navigation can increase survivorship, particular patients and practices may benefit. For instance, low-volume surgeons have shown higher rates of failure at 8 years [49].
11.2.6 Fracture Risk and Notching
Pin site fractures occur at an estimated rate of 1 % with the use of computer-assisted navigation [50]. Risk factors include female sex [50], osteoporosis [51–53], bicortical pin placement [50, 51], and thermonecrosis of the bone [54, 55]. Additionally, computer-assisted TKA is associated with a risk of anterior femoral notching [52, 56, 57]. The association between periprosthetic fracture and anterior femoral notching remains speculative [58, 59], but caution should be enforced for at-risk patients [60].
11.3 Patient-Specific Instrumentation
Patient-specific instrumentation generates disposable cutting blocks specific to the patient’s knee morphology. Preoperative CT scans or MRIs are used to create a 3-D model. The surgeon can then review a proposed preoperative plan. After approval, final recommendations are sent to the manufacturer, and cutting blocks are available usually within 4 weeks. Patient-specific blocks are used for the distal femoral and proximal tibial cuts, while conventional instruments are utilized to complete the remaining procedure. Execution of the plan requires careful intraoperative positioning of the PSI. Since CT-based models do not account for cartilage and soft tissues, exposure of the bony contact points is necessary. Abdel et al. recently showed that utilizing navigation to confirm placement of the PSI is inaccurate [61].
11.3.1 Component Alignment
Recently, two meta-analyses compared PSI and conventional instrumentation and showed similar mechanical axis alignment [62, 63]. Sub-analysis restricted to RCTs also showed no difference in the rate of malalignment [63]. While coronal alignment of femur was not significantly different, PSI did have a lower percentage of malalignment [63, 64]. On the other hand, coronal alignment of the tibial component showed inconsistent results across the studies [63, 64]. Neither a large systematic review nor two meta-analyses found a significant difference in sagittal alignment or the rate of outliers between the two techniques [62–64]. The far majority of studies report no difference in femoral rotation between the two techniques [65, 66], and meta-analysis indicates no difference in the rate of outliers more than 3° from target rotation [64]. Lastly, few differences in postoperative alignment have been noted between CT- and MRI-based PSIs [67]. However, two recent studies show higher precision with MRI-based PSIs [68, 69].
11.3.2 Patient Outcomes
Improved patient outcomes are always a goal with new technology. However, most reports for patient outcomes with PSI are only at 2 years, and the results remain inconclusive [70, 71]. Anderl et al. retrospectively evaluated 108 conventional and 114 PSI total knees, showing no difference in KSS or visual analog pain scores in the short term [70]. One RCT compared 47 conventional TKAs and 48 PSI TKAs at least 6 months postoperatively [71]. KSS were comparable between groups. In addition, Abdel et al. showed similar gait parameters between conventional and PSI TKAs at short-term follow-up [72]. Longer-term RCTs are needed.
11.3.3 Cost-Effectiveness
Cost-effectiveness fails to support PSI [73, 74]. Efficiencies are marginal with a reduction in operative times of 5–12 min [75–77] and 90 fewer minutes for surgical tray processing [73]. Combined, both these factors contribute to cost savings of approximately $322 [73]. However, preoperative imaging ranges from $430 to $1,360, which is in addition to the PSI (approximately $500) [74].
While shorter operative times and faster tray processing can improve operative efficiency, an enormous effort is shifted to the preoperative period. Furthermore, intraoperative plans are subject to change even after the extensive planning [66, 71, 78, 79]. For instance, one study found that the surgical plan predicted component size in less than 50 % of tibias and 25 % of femurs [78]. Moreover, Victor et al. showed malalignment greater than 3° in any plane warranted PSI to be abandoned in 22 % of cases, and preoperative plans were modified in 28 % of cases [79].
11.3.4 Extra-articular Deformities and Intramedullary Obstruction
Similar to navigation, TKA in the setting of extra-articular deformities or obstruction of the intramedullary canal can benefit from PSI. The largest series to date (10 patients) showed excellent results at 3.4 years postoperatively [80].
11.4 Robotic-Assisted Surgery
Robotics add to CAS, assuming some of the operating responsibilities. Semi-active systems, such as the MAKO (formerly MAKO Surgical Corporation, Fort Lauderdale, FL; now Stryker, Mahwah, NJ), allow the surgeon full control of a robotic arm but prevent any deviations from the virtual operative plan. Active systems, such as ROBODOC (Integrated Surgical Systems Davis, Sacramento, CA), can assume responsibility for parts or potentially all aspects of the procedure. The nuances of soft tissue management require more sophisticated models to be developed in the future. At the current time, excessive costs and minimal long-term studies have limited the use of robotics in TKA.
11.4.1 Alignment
RAS shows high accuracy and precision for component alignment [81–83]. Park and Lee [81] prospectively randomized 32 patients to RAS using the ROBODOC system and 30 knees to conventional TKA. While mechanical alignment did not differ between the groups, tibial and femoral implant positions were significantly improved in the coronal and sagittal planes. Song et al. reported similar results in their prospective study of bilateral TKAs [82]. The RAS group had significantly fewer outliers for the mechanical coronal femoral and sagittal tibial alignments. Also, overall mechanical alignment and sagittal femoral alignment more closely approximated surgical goals. The same authors randomized total knee patients and showed a greater number of mechanical axis outliers using conventional instrumentation [83]. Interestingly, the RAS group was able to achieve adequate gap balancing more often than conventional instrumentation. In another RCT, Liow et al. [84] highlighted the ability of robotic surgery to not only minimize mechanical axis outliers, but also to restore the joint line more accurately than conventional surgery. Rotational alignment has limited study, while some suggest more accurate alignment of the femoral component [85].
11.4.2 Outcomes and Complications
Short- and midterm outcomes have been reported for the ROBODOC system, but were not significantly different than for conventional arthroplasty [82, 83]. At 1 year of follow-up, Song et al. found that the WOMAC and HSS scores were similar between both groups in their randomized controlled trial (RCT) [82]. At a follow-up of 5.4 years, there were no differences seen between the conventional and RAS groups for HSS and WOMAC scores [83]. No differences in complication rates have been reported either [83].
11.4.3 Costs
Costs associated with RAS include fixed costs (equipment purchase and maintenance fees) as well as variable costs (advanced preoperative imaging and cleaning fees). Start-up costs are upward of $800,000 [84]. Operating costs contribute as well, quoted at over $1,200 per case [84]. Operative times are variable owing to the steep learning curve. Once surmounted, RAS requires an additional 25 min compared to conventional TKA [82, 83]. Using cost estimates from DeHaan et al. [74], this translates into an additional cost of $1,625 per surgery ($65/min of operative time).
11.5 Conclusions
TKA has dramatically enhanced the management of end-stage knee arthritis. Despite advances over the last few decades, some patients continue to be dissatisfied with their arthroplasty. Component alignment has become a focus, as malalignment has persisted despite improvements in surgical technique. While technology such as navigation, patient-specific instrumentation (PSI), and robotic-assisted surgery (RAS) may enhance surgical execution, it remains unclear whether more accurate alignment can actually improve survivorship or patient satisfaction. At the current time, navigation, PSI, and RAS are more costly than conventional techniques. In order for more widespread adoption, incremental clinical gains and enhanced cost-effectiveness must be demonstrated. There are, however, specific cases where advanced technology has a role such as severe extra-articular deformities and obstruction of the femoral canal.
References
1.
2.
3.
Lotke PA, Ecker ML (1977) Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am 59:77–79PubMed
4.
Bargren JH, Blaha JD, Freeman MA (1983) Alignment in total knee arthroplasty. Correlated biomechanical and clinical observations. Clin Orthop Relat Res 173:178–183PubMed
5.
Jeffery RS, Morris RW, Denham RA (1991) Coronal alignment after total knee replacement. J Bone Joint Surg Br 73:709–714PubMed
6.
7.
8.
9.
Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K (2007) Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty 22:1097–1106PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








