Key points
- •
Noninvasive brain stimulation is typically paired with unilateral therapies of the upper limb.
- •
Many recent clinical trials have failed to augment rehabilitative outcomes, especially for patients with greater motor impairments.
- •
Bilateral therapies may offer a more feasible and neurophysiologic advantage over unilateral therapy to augment rehabilitative outcomes for patients with greater motor impairments.
- •
Based on mechanisms of recovery, this article discusses how to create noninvasive brain stimulation paradigms that are tailored to the individual type of therapy (unilateral or bilateral) across varying degree of impairments.
Introduction
Stroke is a leading cause of long-term adult disability. Although current rehabilitation strategies carry promise, gains are modest where approximately 60% to 80% of survivors continue to experience motor impairments of the upper limb well into the chronic phase of recovery. One reason for the modest recovery of upper-limb function is the diminishing access to rehabilitation, where therapists are required to administer best practice in a limited number of sessions. To address this limitation, current research emphasizes the need for maximizing and accelerating outcomes of rehabilitation within a limited amount of time.
To augment rehabilitative benefits, use of noninvasive brain stimulation (NIBS) has become a popular topic of research. Specifically, NIBS has the potential to augment mechanisms of plasticity that underlie rehabilitation-related recovery. The most commonly used forms of NIBS in research include repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). rTMS operates by using electromagnetic induction, wherein an insulated coiled wire is placed on the scalp. Then, at varying frequencies, the coil produces a brief and strong alternating current that induces a perpendicular spatially focused magnetic field. The magnetic field induces current, which passes unimpeded through the skull, resulting in depolarization of neurons in superficial cortices. High-frequency pulses (≥5 Hz) are used to facilitate excitability of the targeted cortices, whereas low-frequency pulses (<1 Hz) inhibit excitability of the underlying cortices. Unlike rTMS, tDCS applies current directly to the targeted regions and has emerged as a popular NIBS approach because it is simple and easy to use in conjunction with physical and occupational therapy. Using a constant current stimulator, surface electrodes placed in saline-soaked sponges deliver low-levels of direct current (0–4 mA) to the scalp and create changes in cortical excitability. Early animal studies have shown that tDCS modulates neuronal membrane potentials in the cortices, such that anodal tDCS depolarizes membrane potentials and cathodal tDCS hyperpolarizes membrane potentials. As such, anodal tDCS is typically considered excitatory for the targeted region, whereas cathodal tDCS is considered inhibitory. Although the exact mechanisms are unclear, a similar directional change in excitability has been achieved in humans. Nitche and Paulus have shown that anodal tDCS increases excitability and cathodal tDCS decreases cortical excitability. Based on pharmacologic studies, the likely mechanism in humans involves up-regulation of N -methyl- d -asparate receptor activity and modulation of γ-aminobutyric acid (GABA)ergic neuronal activation. Thus, tDCS modulates excitability and spontaneous firing rate of neurons.
The primary application of NIBS approaches in rehabilitation has involved their pairing with unilateral upper-limb therapies. Such therapies focus on intensively retraining the paretic limb and restraining or otherwise discouraging movement of the nonparetic limb. Examples include constraint-induced movement therapy, unilateral task-oriented practice, or learning involving only the paretic limb, among several others. NIBS approaches are applied before therapy (rTMS) or during therapy (tDCS).
Despite promising early studies, NIBS has shown somewhat limited effects to augment rehabilitative outcomes of the unilateral upper limb in more recent and larger clinical trials. Hence, its use remains for the most part investigational. In trying to understand the failures, it seems that the benefits of NIBS plus therapy are modest, and vary considerably from patient-to-patient, failing especially in patients with greater motor impairments. Thus, this article addresses important lingering questions that may help devise the best combinations of NIBS with rehabilitative therapies. Are mechanisms that NIBS seeks to entrain in its pairing with unilateral therapy generalizable across patients in all ranges of impairment, or do these mechanisms fail across patients with greater motor impairments? In such cases, are therapies targeting alternate mechanisms better suited for the more impaired instead?
Several groups have recently suggested the importance of bilateral behavioral therapies as alternates to unilateral upper-limb therapies, including bilateral arm training with rhythmic auditory cueing (BATRAC), bilateral isokinematic training, active-passive bilateral training (APBT), and contralaterally controlled functional electrical stimulation (CCFES). Even though there is no direct evidence concluding whether they are better than unilateral therapies across certain ranges of severity, it is generally considered that they likely could be more efficacious for patients with greater motor impairments, because many of the previously mentioned therapies enable the nonparetic limb to drive movement of the paretic limb. However, there is limited understanding of what mechanisms underlie bilateral therapies, which is why there is lack of discussion on how to pair NIBS with bilateral therapies. In contrast, the mechanisms underlying unilateral therapies are better understood, which is why there is considerable evidence discussing how to apply NIBS to affect outcomes of unilateral therapies. The aim of this article is to (1) compare possible mechanisms of recovery that may be engaged by unilateral and bilateral therapies, (2) explain potentially how these mechanisms may vary across ranges of damage and impairment, and (3) present a theoretic framework for how to create NIBS paradigms that are tailored to distinctly augment bilateral and unilateral therapies.
Introduction
Stroke is a leading cause of long-term adult disability. Although current rehabilitation strategies carry promise, gains are modest where approximately 60% to 80% of survivors continue to experience motor impairments of the upper limb well into the chronic phase of recovery. One reason for the modest recovery of upper-limb function is the diminishing access to rehabilitation, where therapists are required to administer best practice in a limited number of sessions. To address this limitation, current research emphasizes the need for maximizing and accelerating outcomes of rehabilitation within a limited amount of time.
To augment rehabilitative benefits, use of noninvasive brain stimulation (NIBS) has become a popular topic of research. Specifically, NIBS has the potential to augment mechanisms of plasticity that underlie rehabilitation-related recovery. The most commonly used forms of NIBS in research include repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). rTMS operates by using electromagnetic induction, wherein an insulated coiled wire is placed on the scalp. Then, at varying frequencies, the coil produces a brief and strong alternating current that induces a perpendicular spatially focused magnetic field. The magnetic field induces current, which passes unimpeded through the skull, resulting in depolarization of neurons in superficial cortices. High-frequency pulses (≥5 Hz) are used to facilitate excitability of the targeted cortices, whereas low-frequency pulses (<1 Hz) inhibit excitability of the underlying cortices. Unlike rTMS, tDCS applies current directly to the targeted regions and has emerged as a popular NIBS approach because it is simple and easy to use in conjunction with physical and occupational therapy. Using a constant current stimulator, surface electrodes placed in saline-soaked sponges deliver low-levels of direct current (0–4 mA) to the scalp and create changes in cortical excitability. Early animal studies have shown that tDCS modulates neuronal membrane potentials in the cortices, such that anodal tDCS depolarizes membrane potentials and cathodal tDCS hyperpolarizes membrane potentials. As such, anodal tDCS is typically considered excitatory for the targeted region, whereas cathodal tDCS is considered inhibitory. Although the exact mechanisms are unclear, a similar directional change in excitability has been achieved in humans. Nitche and Paulus have shown that anodal tDCS increases excitability and cathodal tDCS decreases cortical excitability. Based on pharmacologic studies, the likely mechanism in humans involves up-regulation of N -methyl- d -asparate receptor activity and modulation of γ-aminobutyric acid (GABA)ergic neuronal activation. Thus, tDCS modulates excitability and spontaneous firing rate of neurons.
The primary application of NIBS approaches in rehabilitation has involved their pairing with unilateral upper-limb therapies. Such therapies focus on intensively retraining the paretic limb and restraining or otherwise discouraging movement of the nonparetic limb. Examples include constraint-induced movement therapy, unilateral task-oriented practice, or learning involving only the paretic limb, among several others. NIBS approaches are applied before therapy (rTMS) or during therapy (tDCS).
Despite promising early studies, NIBS has shown somewhat limited effects to augment rehabilitative outcomes of the unilateral upper limb in more recent and larger clinical trials. Hence, its use remains for the most part investigational. In trying to understand the failures, it seems that the benefits of NIBS plus therapy are modest, and vary considerably from patient-to-patient, failing especially in patients with greater motor impairments. Thus, this article addresses important lingering questions that may help devise the best combinations of NIBS with rehabilitative therapies. Are mechanisms that NIBS seeks to entrain in its pairing with unilateral therapy generalizable across patients in all ranges of impairment, or do these mechanisms fail across patients with greater motor impairments? In such cases, are therapies targeting alternate mechanisms better suited for the more impaired instead?
Several groups have recently suggested the importance of bilateral behavioral therapies as alternates to unilateral upper-limb therapies, including bilateral arm training with rhythmic auditory cueing (BATRAC), bilateral isokinematic training, active-passive bilateral training (APBT), and contralaterally controlled functional electrical stimulation (CCFES). Even though there is no direct evidence concluding whether they are better than unilateral therapies across certain ranges of severity, it is generally considered that they likely could be more efficacious for patients with greater motor impairments, because many of the previously mentioned therapies enable the nonparetic limb to drive movement of the paretic limb. However, there is limited understanding of what mechanisms underlie bilateral therapies, which is why there is lack of discussion on how to pair NIBS with bilateral therapies. In contrast, the mechanisms underlying unilateral therapies are better understood, which is why there is considerable evidence discussing how to apply NIBS to affect outcomes of unilateral therapies. The aim of this article is to (1) compare possible mechanisms of recovery that may be engaged by unilateral and bilateral therapies, (2) explain potentially how these mechanisms may vary across ranges of damage and impairment, and (3) present a theoretic framework for how to create NIBS paradigms that are tailored to distinctly augment bilateral and unilateral therapies.
Mechanisms of recovery underlying unilateral therapy
Typically, coordination between limbs requires modulating motor overflow, where motor overflow refers to facilitation from the “moving” cortices to the opposing “resting” hemisphere. During unilateral movement of a limb, mirror movements can occur in the opposite resting limb if motor overflow is not regulated. Interhemispheric interactions conducted via transcallosal pathways between both hemispheres help regulate overflow. Specifically, the hemisphere contralateral to the moving limb imposes an inhibitory influence on the ipsilateral hemisphere, whereas the ipsilateral hemisphere relaxes its counterinhibition to allow for a purely unilateral movement.
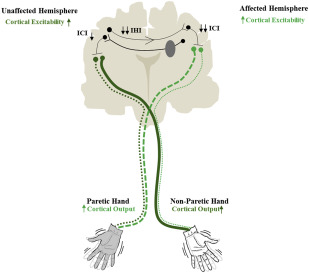
Following stroke, however, the mechanism of regulating motor overflow is disrupted, resulting in a series of events that constitutes what is commonly referred to as the interhemispheric competition model. Based on this model, during unilateral movement of the paretic limb, the affected hemisphere weakly inhibits the unaffected hemisphere to regulate overflow ( Fig. 1 ). In turn, the “disinhibited” unaffected hemisphere overly inhibits the affected hemisphere, further weakening its excitability and the drive to move the paretic limb. Such an imbalance of mutual inhibition presumably exacerbates as patients rely on using their nonparetic limb at the cost of the weak paretic limb. Therefore, the typical recommendation based on the interhemispheric competition model is to unilaterally retrain the paretic limb but restrain or discourage movements of the nonparetic limb. By intensively retraining the paretic limb, it is believed the weak affected hemisphere is facilitated, and effectively counters inhibition from the unaffected hemisphere, promoting gains in recovery of the upper limb.
Combining noninvasive brain stimulation with unilateral therapy based on theory of underlying mechanisms
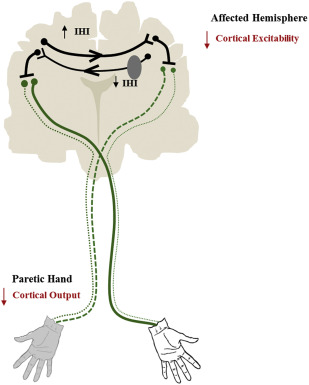
In accordance with the interhemispheric competition model, present-day NIBS approaches aim to upregulate excitability of the affected hemisphere but inhibit that of the unaffected hemisphere to enhance rehabilitative outcomes. Toward this end, multiple research groups have used high-frequency rTMS or anodal tDCS to excite the affected hemisphere or low-frequency rTMS or cathodal tDCS to inhibit the unaffected hemisphere ( Fig. 2 ; for a full review see Hoyer and Celnik or Sandrini and Cohen ). In either hemisphere, the most common target is the primary motor cortex (M1), because evidence suggests its adaptive plasticity is intimately associated with paretic upper-limb recovery.
Limitations of unilateral therapies and associated noninvasive brain stimulation approaches
Larger clinical trials have had limited success when replicating the early promise of pairing NIBS with unilateral therapies. One possible reason for the disappointing results is that the groups were less homogenous and included patients with a wider range of impairment than in earlier smaller studies. Previous studies have discussed that pairing NIBS with unilateral therapy is less effective for the more impaired chronic stroke patients. An important question to consider is whether the model of interhemispheric competition informing unilateral therapies and present-day NIBS approaches in the chronic stroke population is applicable across patients with greater severity. We describe three major reasons for why exciting the affected hemisphere and/or inhibiting the unaffected M1 may generalize poorly across patients with greater severity: (1) heterogeneity of stroke population, (2) extent of damage in the affected hemisphere, and (3) the influence of the unaffected hemisphere.
Stroke Population Heterogeneity
Within the stroke population, such factors as age, location and profile of lesion, and comorbidities all show high variation. Furthermore, many of these factors also contribute to the large variability in overall severity of the motor deficit, with some patients having substantial amounts of movement and others having limited movement in the paretic upper limb. Applying unilateral therapy to patients with limited movement can inherently be challenging. Specifically, because of their inability to use their paretic limb in task-based therapies, severely impaired patients are generally unable to realize the maximum benefits from unilateral therapies, which may explain why they show greater inconsistencies in benefits of NIBS. Most of these patients are unable to meet the minimal movement criteria for participation in unilateral rehabilitation, where often participants are required to have at least 20° of wrist extension and 10° extension of at least two fingers. When severely impaired patients are included in trials, they do not show the same recovery as the less impaired, suggesting that unilateral behavioral therapies may be less successful and feasible for this population. Therefore, current NIBS approaches combined with unilateral therapies generalize poorly across the more impaired patients because the fundamental therapy itself is inconsistently effective for this population.
Damage in the Affected Hemisphere
When patients experience hemiparesis following stroke, those with subcortical lesions typically have damage to the corticospinal tracts. The corticospinal tract originates from the primary and premotor cortices where the descending pathways synapse with lower motor neurons at the level of the spinal cord to execute volitional movements. It has previously been shown with diffusion tensor imaging that patients with poor integrity of corticospinal tracts following stroke exhibit more severe motor impairments. Thus, it is possible that patients, especially those with severe motor impairments, do not have adequate residual corticospinal pathways that can be excited in the affected hemisphere with unilateral therapies or with current NIBS approaches. Hence, they fail to benefit from modulation of interhemispheric mechanisms of recovery for the paretic limb.
Influence of the Unaffected Hemisphere
Several groups, including our own, have demonstrated that the unaffected hemisphere is not always inhibitory to the affected hemisphere as traditionally believed. Rather, it can mediate recovery when substrates in the affected hemisphere are damaged considerably and patients experience greater severity of impairment. Machado and colleagues show that following hemispherectomy, the unaffected hemisphere in rodent models assumes the role of the affected hemisphere, suggesting it becomes critical for recovery. In fact, rodents with large lesions experience a decline in motor function when the unaffected hemisphere is anesthetized. As such, the unaffected hemisphere may provide an adaptive role through ipsilateral pathways originating from the unaffected hemisphere and innervating lower motor neurons devoted to the paretic limb. Carmel and colleagues have recently demonstrated in a rodent model that electrical stimulation applied to facilitate the unaffected hemisphere promotes recovery of skilled forelimb behavior through ipsilateral pathways. Furthermore, Bachmann and colleagues demonstrated in a mouse model that unilateral strokes induces axonal sprouting from the unaffected hemisphere at the level of the brainstem–spinal cord connections, with the possibility to gain control over the affected limb.
The potential adaptive role of the unaffected hemisphere in humans also aligns with these animal studies. For example, when TMS is applied transiently to disrupt the unaffected premotor cortex, patients with greater impairments experience greatest disruption in motor performance of the paretic hand, suggesting that with greater impairment, the likely role of the unaffected cortices becomes more relevant. Furthermore, if NIBS is applied to inhibit the unaffected hemisphere, patients with greater impairments experience a transient decline in upper-limb motor function, suggesting that with greater impairment, unaffected cortices likely offer an adaptive potential for recovery. These studies suggest that the role of the unaffected hemisphere is expressed more so with greater impairment and deficit, where its influence can be considered more adaptive than what is known typically. Although animal studies facilitating the unaffected hemisphere in models of greater damage have recently supported these new views by demonstrating a causal adaptive role of the unaffected hemisphere in chronic recovery, direct evidence as to the total contribution of the unaffected hemisphere in humans remains to be seen.
The heterogeneity of the stroke population, extent of damage of the affected hemisphere, and the influence of the unaffected hemisphere may explain the shortcomings of unilateral therapies and the inconsistencies of the resultant NIBS studies. The generalizability of the theory of interhemispheric competition as a global mechanism of motor recovery following stroke thus becomes questionable. By emphasizing a single mechanism we risk creating augmentative NIBS approaches that lack flexibility to consistently serve the spectrum of stroke patients. In the same vein, we are likely to miss the advantages of potentially high-yielding therapies, such as bilateral behavioral paradigms, that might also promote recovery.
Bilateral therapy as an alternative approach
Bilateral approaches differ from unilateral therapies because they require moving both limbs simultaneously, either independently or in a linked manner. For example, bilateral isokinematic training requires patients to move both limbs, but actions of one are not dependent or controlled by actions of the other. In contrast, Stinear and Byblow’s APBT, Whitall and colleagues’s BATRAC, and Knutson and colleagues’s CCFES link movements of both limbs. Using external instrumentation, such as mechanical fixations or electrical stimulation, the nonparetic limb drives the movement of the paretic limb.
Regardless of the type, bilateral therapies may provide a more feasible alternative to unilateral therapies. Because patients with severe impairments are typically unable to participate in unilateral therapies, bilateral therapies, such as APBT, BATRAC, and CCFES, where movement of the nonparetic limb drives movement of the paretic limb, could potentially provide all patients an opportunity to be involved and benefit from rehabilitation.
Although bilateral therapies may be more feasible, the important question is whether they are more efficacious than unilateral therapies. Overall, results are equivocal and seem to depend on at least the severity of motor impairment and the nature of clinical outcome of interest. For example, in a recent systematic review, van Delden and colleagues concluded that bilateral therapies are as effective as unilateral therapies, but unilateral therapies still offered a slight advantage for functional independence and daily use of paretic hand across patients with mild-to-moderate impairment of the distal upper limb. In contrast, McCombe Waller and Whitall argue that bilateral therapies involving repetitive reaching, as in BATRAC, offer an advantage for patients with moderate-to-severe impairments, at least in terms of proximal strength. Whether unilateral therapies improve independence and use of hand in daily life or bilateral therapies serve as a useful alternative for proximal function, their effectiveness can be best contrasted when the effect of initial impairment is balanced and the clinical goal is carefully considered.
Mechanisms of Bilateral Movement in Chronic Stroke
If indeed bilateral therapies afford greater advantage than unilateral therapies across the moderate-to-severely impaired patients, then this would suggest that their underlying mechanisms are more resilient in the presence of greater damage. Understanding these mechanisms of recovery could help derive a model that supplements the classical theory of interhemispheric competition. Here, we summarize evidence of potential mechanisms underlying bilateral therapies.
First, whether passive or active, bilateral movements could engage both hemispheres. One clear advantage would be that the unaffected hemisphere, now with evidence pointing to its adaptive and compensatory role for the more impaired patients, would naturally become engaged. Recruiting the unaffected hemisphere could indirectly facilitate the weak affected hemisphere because by symmetrically moving both limbs for a common purpose, both hemispheres become coupled. As a result of coupling, Mudie and Matyas explain, the unaffected hemisphere may offer a template of motor network recruitment to the affected hemisphere, allowing the paretic limb to learn from the nonparetic limb. This may be particularly necessary in more impaired patients where the damaged hemisphere has insufficient cortical-corticospinal resources to affect its own movement plans.
However, what is the evidence that a template could be uniquely elicited in bilateral movement? Studies with functional MRI (fMRI) show that bilateral movements elicit unique and greater activation of bilateral primary sensorimotor, premotor, and supplementary motor cortices in comparison with unilateral movements, and that these distinctions amplify with therapy. Patients who show the greatest recovery with BATRAC exhibit the highest gains in fMRI activation in the unaffected hemisphere, especially the unaffected premotor cortex, whereas patients who experience greater functional recovery with unilateral therapy exhibit greater activation of the premotor cortex of the affected hemisphere. Therefore, fMRI activation demonstrates that substrates recruited in bilateral movement are extensive and bihemispheric, compared with those recruited in unilateral movement.
Still, fMRI evidence alone may not be able to verify that a template of learning indeed transfers from one hemisphere to the other during bilateral movement. Studies that assess the neural basis of motor planning or functional and effective connectivity between hemispheres are needed for confirmation. As an example, TMS could reveal the neurophysiologic substrates underlying a transfer. Following stroke, one conceivable outlet for coupling and transfer of learning could involve mutual disinhibition of both hemispheres ( Fig. 3 ). Bilateral movements are in a unique position to potentiate disinhibition unlike unilateral movements because bilateral symmetric movements are considered natural and the default state of interlimb coordination. Therefore, with bilateral synchronous movements, there is a decrease in intracortical inhibition within M1 and interhemispheric inhibition between M1s as demonstrated with TMS. Release of inhibition could facilitate excitability of corticospinal output from the affected M1 and help restore the balance of mutual interhemispheric inhibition. Thus, it is possible that synchronous somatosensory feedback in bilateral motion, and a single set of motor commands linking bimanual movements may help upper limbs to become functionally coupled, and both hemispheres to release their inhibition on one another to allow transfer and exchange of learning.