CHAPTER 20
Surgery in the Management of Spasticity
David A. Fuller
PHILOSOPHY OF SURGERY FOR SPASTICITY
Spasticity occurs as a result of injury to the upper motor neurons of the central nervous system (CNS). Existing surgical techniques do not repair the injury to the CNS, but rather aim to improve or modulate the output of the CNS to improve a person’s function. Surgical intervention may be targeted at the brain, spinal cord, peripheral nerves, or musculoskeletal system (Table 20.1). The goals of this chapter are for the reader to gain understanding of surgery for spasticity, the surgical options for spasticity, and the controversies within this evolving field.
There are two fundamental questions that anyone treating spasticity must consider:
1. Should surgery be considered as a treatment option?
2. And if surgery is believed to be the best option, then what surgery should be done?
The answer to question 1 is a resounding “yes” for any patient with spasticity. Although certainly surgery is not always necessary for spasticity, it must always be considered as an option for anyone taking a holistic approach to the problem. Surgery is unfortunately often viewed as the treatment of last resort, when all else has failed. This view is narrow minded and may do a disservice to a patient for whom surgery is really the best and only option to effect change for a deformity driven by spasticity. A limited perspective toward surgery for spasticity unfortunately remains pervasive for many reasons including lack of knowledge, perhaps unfavorable past experiences, and lack of access to surgery as a treatment for spasticity. Few physicians have expertise in surgery for spasticity, which compounds the issue. Surgery as a treatment for spasticity has a proven track record of success over decades and must always be considered as an option.
The answer to question 2 will be discussed throughout the rest of this chapter. Controversies exist regarding the indications for surgery, the timing of surgery, and even what type of surgery to perform. And patients with spasticity may have many comorbidities and unique extenuating circumstances that make it difficult to form generalizations regarding the role of surgery for spasticity. Decisions regarding surgery are often best made through a patient-centered team approach, which may include the patient, family, therapist, nutritionist, nurse, counselor, physiatrist, surgeon, and even others contributing to an oftentimes difficult decision to proceed with surgery. This chapter in general focuses on adults with spasticity rather than children with immature neurologic and musculoskeletal systems for whom entire texts have been dedicated. Many of the principles remain the same, whether treating adults or children with spasticity.
HISTORY OF SURGICAL INTERVENTIONS FOR SPASTICITY
Historically, surgery has been targeted at the neurologic system or the musculoskeletal system, which is a challenge because few surgeons are facile at operating on both systems. For the neurologic system, three techniques have been employed including brain surgery, spinal cord surgery (rhizotomy), and peripheral nerve surgery (complete or partial neurectomy). Each of these neurologic surgical techniques has a unique role to play in very specialized patient populations. Brain surgery utilizing temperature control electrocoagulation and cerebellar stimulation has been attempted with poor results (1–3). The most popular form of spinal cord surgery for spasticity is selective posterior rhizotomy. Typically, posterior rhizotomy is done for patients with cerebral palsy (CP) who have pure spasticity, good strength and motor control, minimal fixed contractures, and good intelligence. Posterior rhizotomy is currently used with variable success in limited patients (4,5). Posterior rhizotomy can, however, have long-lasting favorable results (6). Neurectomy, a complete cutting of a peripheral nerve, has been used with some success for very specific problems (7). Neurectomy, particularly of mixed motor and sensory nerves, can have the unfortunate consequence of permanent painful dysesthetic pain (8). Partial neurectomy has recently been reported with success for spasticity within both the upper and lower extremities (9,10). The majority of successful surgical interventions for the management of adult spasticity are currently performed on the peripheral muscles and tendons (11) and are the primary focus of this chapter, as techniques and examples of musculoskeletal surgery are discussed in detail.
TABLE 20.1
SURGERY FOR SPASTICITY | |
Target Organ | Procedure |
Brain | Stereotactic neurosurgery |
Spinal cord | Posterior rhizotomy |
Peripheral nerve | Neurectomy |
Muscle or tendon | Lengthening |
Spasticity is a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex (12–17). The result manifested in the musculoskeletal system is altered limb function. In the upper extremities, spasticity affects one’s ability to interact with the environment and the performance of activities of daily living is impaired. In the lower extremities, the greatest impact of spasticity is noted in ambulation and positioning of the legs. Surgery for spasticity is directed at correcting these deficits. Sometimes it can be highly effective, providing permanent correction. Other times, the procedure is not successful, perhaps even worsening a difficult situation. To be an appropriate option, the surgery needs to be as effective, predictable, permanent, and as painless as possible. The more that is known about the pathology of the upper motor neurons, the better the potential outcome of surgery aimed to improve or modulate the output of the upper motor neurons.
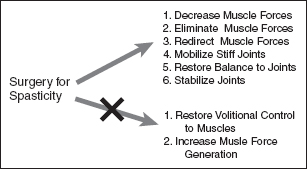
FIGURE 20.1 Although surgery cannot increase the force that a muscle generates or impart volitional control to a muscle with no underlying motor control, it can sometimes unmask motor control in a muscle that may not have been evident initially.
Surgery can alter the force that a muscle exerts, either by decreasing, redirecting, or eliminating the muscle force (18,19). Surgery can also increase a muscle’s effect by eliminating or decreasing the force from an antagonist muscle (20). Mobilization of stiff joints resulting from spasticity by removing passive restraints to movement such as heterotopic ossification, adhesions, or contracted ligaments can also be treated in the operating room. Although surgery cannot increase the force that a muscle generates, nor impart volitional control to a muscle with no underlying motor control, it can sometimes unmask motor control in a muscle that may not have been evident initially (Figure 20.1).
Surgery should be thought of as an important component of the paradigm for the management of the upper motor neuron syndrome. As a result, it has an important place in a comprehensive spasticity management program. Even after surgical intervention, there is still often a need for other treatment interventions including physical therapy, oral medications, chemodenervation, chemoneurolysis, and intrathecal medications. The challenge for clinicians is to pick the best modality or combination of them to treat the problems related to a person’s spasticity. Each available treatment for spasticity has unique benefits, costs, and risks. Surgery has historically been viewed as a last resort, but this view is now antiquated. For some patients, especially those with a focal presentation, surgical intervention should be considered earlier, perhaps as the first treatment.
For some patients, an operative procedure has many advantages over many of the other treatment modalities available for spasticity, and for some patients, it may be considered ahead of other modalities. In the right patient, when properly planned and performed by a skilled clinician, the results are predictable as well as permanent, which is in contrast to many other interventions that are commonly employed. In this chapter, we provide examples of proven surgical techniques to familiarize the reader with conditions amenable to surgical treatment. The primary focus is on principles of surgical management, which is discussed first, and is then followed by specific surgical procedures. Spasticity is encountered in many disorders including traumatic brain injury (TBI), stroke (cerebrovascular accident [CVA]), CP, and multiple sclerosis (16,21) The principles discussed in this chapter are applicable to all of these disorders in all age ranges.
GOALS OF SURGERY FOR SPASTICITY
The goals of surgery for spasticity are no different from those of nonsurgical treatment. Some goals focus on improving function through improving active movement. These goals are classified as active goals. Examples of these include better forward flexion of the shoulder for grooming, better opening and closing of the hand for grasping and releasing objects, or an improved overall gait. In other cases, where patients have more advanced spasticity and demonstrate no or very limited active movement, the goals consist of passive functional improvement and the treatment addresses improving passive function and passive movement. Examples of these include increase ease for the patient or caregiver to position the patient or limb, easier hip abduction for perineal care, improved elbow extension for ease of dressing, or easier finger extension for improved hygiene of the palm (22).
Other goals of surgery for spasticity include pain relief, improved cosmesis, decreased reliance on systemic medication, or chemodenervation. In regard to pain, spastic muscles can be a source of pain, which can be addressed by eliminating the spasticity (23). Cosmesis can be an important issue to a person’s self-esteem and quality of life; surgical management of spasticity can address many of the balance issues and aesthetics of the limb and person. Sedation is a side effect of many of the oral medications prescribed for the treatment of spasticity. Sedating medications can exacerbate the already impaired sensorium of a person with TBI or CVA and they can also hinder recovery. Many of the interventions for the management of muscle overactivity have a limited duration of effect. Physical therapy and serial casting may be very effective at stretching out a mildly contracted joint, but once the treatment is stopped, the deformity often returns. Chemodenervation with the botulinum toxins can provide treatment for muscle overactivity, but the results last months and not a lifetime (Table 20.2). Muscle overactivity can also exacerbate other conditions. Examples of this include exacerbation of posttraumatic elbow osteroarthrosis by increased elbow tone, or exacerbation of median nerve compression and carpal tunnel syndrome as a result of flexor spasticity at the wrist and/or finger flexors (24).
TABLE 20.2
SURGICAL GOALS |
1. Improved function • Active function • Passive function 2. Pain relief 3. Decreased reliance on systemic medications 4. Permanent solution rather than temporizing treatment 5. Improved cosmesis |
FACTORS IMPORTANT IN THE DECISION-MAKING PROCESS
Surgery is indicated when nonoperative options have failed. It may also be indicated when nonsurgical options can be expected to fail, such as a rigid, long-standing equinus contracture at the ankle (Figure 20.2). Attempts at managing a rigid contracture with physical therapy or chemodenervation would not be successful and would be inadvisable for a number of reasons. These futile treatments would consume valuable resources, require time, potentially promote prolongation and exacerbation of the deformity, and, with a potentially high risk/benefit ratio, potentially hurt the patient without a realistic expectation of clinical improvement. An operation to address equinus deformity is safe, effective, and cost-effective and should be the treatment of choice for such cases (25).
Medical stability is important for a person to safely undergo a procedure and is an important factor in the decision to take a person to the operating room. Some patients with spasticity are healthier than others. Often people with TBI-related spasticity are younger and healthier than those with spasticity from CVA. Preoperative evaluation should ensure that the patient has adequate vascularity to heal lower extremity surgical wounds. In addition, a cardiovascular evaluation is usually necessary before operating on a patient with a prior CVA. A person’s medication regimen must be carefully reviewed when planning surgery, management of diabetic treatment and anticoagulants needs to be considered and managed appropriately perioperatively.
The sensory component of a person’s deficits is often overlooked in the assessment of spasticity and the development of a treatment program. Profound sensory loss can significantly limit functional improvement even after the motor control issues are addressed successfully. This is true for both the upper and lower extremities. In the upper extremity, severe sensory loss may prevent a patient from using the limb even if he or she is capable. Similarly, in the lower extremity, sensory deficit may put the patient at risk for skin ulceration with increased weight bearing and no change in gait function. Profound sensory loss can be a contraindication to either upper or lower extremity surgery.
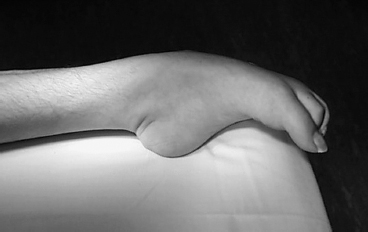
FIGURE 20.2 A severe, long-standing equinus contracture due to spasticity such as this is unlikely to respond to nonsurgical treatment.
Appropriate Timing for Intervention
Selecting the appropriate time for a surgical intervention for spasticity is critical as operating too early or too late can lead to inferior outcomes. Some variables influencing the timing of surgery are specific to a patient such as age, overall health, motivation, and cognition. Other considerations are procedure specific. An operation to rebalance soft-tissue structure is a good demonstration of this concept. It will only be successful if the underlying joints are supple, which may only exist for a short period after the onset of spasticity. Surgical arthrodesis, on the other hand, can be expected to yield good results even when performed for a long period after onset. Figure 20.3 demonstrates a person with a significant equinovarus deformity involving the right foot. Addressing this condition early on will ensure greater success and enable the person to begin ambulating more comfortably earlier.
For many patients with spasticity, an injury occurs to the CNS at a discreet point in time, regardless of etiology. After the initial event, a period of neurologic recovery occurs, which can potentially continue for years. At some point in the recovery, a plateau is reached. However, in some patients even before reaching this plateau, the manifestations of spasticity and its effect on their function are known. A pattern of movement, impairment, and disability becomes evident as demonstrated in Figure 20.4; surgery should only be contemplated after it is apparent what pattern will result after neurologic recovery is nearly complete.
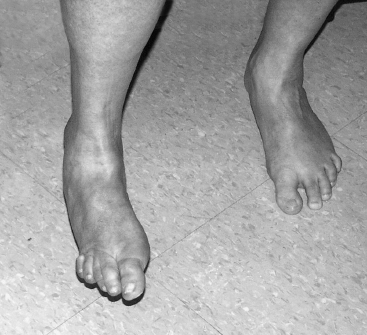
FIGURE 20.3 A spastic equinovarus deformity with associated cavus and claw toes prevents comfortable weightbearing and gait. Patients often desire return to function as quickly as possible. Delay of treatment will prolong disability. A single surgery can predictably and permanently correct this deformity.
Performing surgery is not indicated when there is a reasonable likelihood that the spasticity and disability will resolve with time. Complicating this issue is that the natural history of recovery is not always known early after the injury, and therefore a period of observation is appropriate. During this period of early recovery when surgery is relatively contraindicated, observation and temporizing treatments are essential to maintain supple joints and surgery. For many patients, this dynamic period will last about 6 months or perhaps a little more. During this period, extensive rehabilitation and recovery can take place and multimodal nonsurgical treatments are indicated.
In many patients, approximately 6 months after the initial event, the examination and pattern of spastic muscles will begin to stabilize (Figure 20.2). Surgery can be contemplated at this point depending on other patient-specific issues. Some of these issues include nutrition, pain tolerance, cognition, ability to cooperate with rehabilitation, and comorbidities such as cardiovascular disease. Some operations require more patient cooperation and rehabilitation afterward than others. If the patient is not capable of participating in postoperative rehabilitation, then surgery may need to be delayed. Malnutrition, poor skin integrity, and poor pain control may be other relative contraindications to surgery. Significant vascular disease may also preclude surgery, particularly on the lower extremities where wound healing may be compromised.
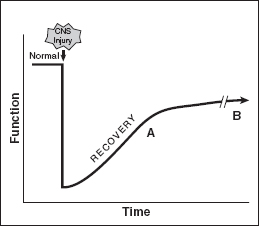
FIGURE 20.4 An example of functional recovery is shown graphically after an injury to the CNS. If functional recovery is incomplete, a plateau is reached at some point in time. At the time point A that a plateau of recovery is beginning, surgery can be done to recover additional function. Surgery can also be done later at time B, but surgery should not be delayed too long. Typically, surgery should be done between 6 and 18 months after the injury to the CNS. CNS, central nervous system.
Conversely, delaying an operation for too long may also be problematic and limit the best outcome. The result of waiting for surgery may in some cases do nothing more than increase and/or prolong a patient’s disability and suffering. It is also possible that by letting too much time pass, the performance of the procedure may make it more difficult if not impossible to perform. Many of the interventions for spasticity involve rebalancing the muscle forces around a joint. If the activity has been out of balance for a prolonged period of time, then it is possible that rigid contractures would have developed in the joint precluding attempts at rebalancing. Spasticity at the foot/ankle serves as an excellent example of this issue. Waiting too long for the treatment of equinovarus deformity may result in a contracture that would have responded well to tendon rebalancing earlier in recovery, but may only be amenable to an ankle arthrodesis later in recovery. Table 20.3 highlights advantages and disadvantages of early and late surgeries. Advanced, rigid deformities also put neurovascular structures at higher risk when a surgical procedure stretches a contracted joint. Waiting too long to release or lengthen the hamstrings can allow the popliteal artery to contract, and attempts to straighten the knee late in recovery will stretch the artery beyond its limits, possibly leading to ischemia in the leg. A patient with advanced knee flexion contracture is shown in Figure 20.5, where the window of opportunity for effective soft-tissue releases has been missed.
TABLE 20.3
TIMING OF SURGERY | |
Early Surgery (Time Point A in Figure 20.4) | Later Surgery (Time Point B in Figure 20.4) |
Advantages | Advantages |
• Supply joints • Shorter duration of disability | • Natural history of recovery more clearly known • Greater healing of initial injury |
Disadvantages | Disadvantages |
• Neurologic condition may be dynamic and unpredictable • Medical morbidities and initial injury are relatively recent | • Stiffer joints • Longer disability |
Typically, the ideal timing for surgery for spasticity is usually between 6 and 18 months after the injury to the CNS; however, it is occasionally done earlier in some extreme cases that do not respond to nonsurgical treatment. A procedure can also be performed later than 18 months after injury with good results but the 6- to 18-month time frame is a good general guideline. The decision to take a patient to the operating room should be made after input from all of the clinicians and other stakeholders. This includes the patient, family, the physiatrist, neurologist, therapists, nurses as well as the surgeon.
MYOTENDINOUS SURGICAL TECHNIQUES FOR TREATMENT OF SPASTICITY
Spasticity is in part due to the stretch reflex of the muscle. A principle of surgery for spasticity is to lengthen the spastic muscle–tendon unit, therefore decreasing the tension in the muscle and hence decreasing the spastic stretch response. By lengthening a muscle or tendon, the tension in the intrafusal muscle spindle is decreased and the stretch reflex is diminished (7,12). Different techniques have evolved to effectively lengthen muscles (Table 20.4). Although reduction in muscle tone and improved joint mobility and function can be expected with lengthening, the overall electromyographic (EMG) patterns of surgically treated muscles may remain unchanged (26). The most extreme lengthening would simply be a release of the muscle–tendon unit, but then all hope of active function from this myotendinous structure would be lost. Figure 20.6 shows a hand with spasticity causing lack of full finger extension in the ulnar aspect of the hand. To achieve improved finger extension, surgical intervention could either lengthen the tendons of the fingers or advance/slide the muscle origins distally to increase the active extension of the fingers. These surgical techniques are discussed in this section.

FIGURE 20.5 A patient with advanced flexion contractures of the hips and knees. Passive function is severely compromised. Sitting posture, donning clothing, and hygiene are impossible in this patient. Too much time has passed for effective soft-tissue releases to treat the spasticity in this patient.
TABLE 20.4
SURGICAL LENGTHENING TECHNIQUES |
1. Fractional lengthening |
2. Muscle slide or advancement |
3. V-to-Y lengthening |
4. Z-lengthening |
5. Hemitenotomy lengthening |
The most valuable and versatile lengthening technique in spasticity surgery is called a fractional lengthening. Most muscles have a region wherein there exists overlap between the muscle and the tendon. At this level, where there is overlap between the muscle and tendon, the fractional lengthening is performed. The tendon is simply transected within the substance of the muscle. The muscle at the myotendinous junction is then able to stretch in the region where the tendon was cut, allowing overall lengthening of the structure. Clinical pictures of a fractional lengthening of a superficial finger flexor in the forearm are shown in Figure 20.7.

FIGURE 20.6 Lack of full finger extension in the ulnar aspect of the hand is seen in this patient who had a stroke 3 years previously. The patient is having difficulty with active function and wishes to be able to extend her fingers more easily to improve her active function. Different surgical techniques exist to relax the finger flexor spasticity and improve active extension. Fractional lengthening of finger tendons is a commonly employed surgical technique to improve spastic finger flexion deformity.
The amount of lengthening that occurs with a fractional lengthening will be proportional to the amount of tension in the muscle or the amount of passive stretch that is applied. This is depicted in Figure 20.8 using a spring mechanism analogy. In a muscle with greater spasticity, more lengthening will occur, whereas a muscle with less spasticity will have a smaller amount of lengthening. This allows the intrinsic spasticity of the specific muscle to determine the amount of lengthening that will occur. If greater passive stretch is applied either intraoperatively or postoperatively with rehabilitation, then greater lengthening will also occur. If there is not adequate muscle surrounding the tendon, then it is possible for the myotendinous junction to overlengthen and rupture.
After some period of time (approximately 3 months) after a fractional lengthening has been performed, a new tendon will heal, filling in the gap that was created with the surgery. Before the tendon gap is filled with new tendon, the lengthening can be increased with passive stretch of the muscle or due to the spasticity. During this period of rehabilitation, it is important not to overstretch the muscle and excessively weaken or rupture the muscle.
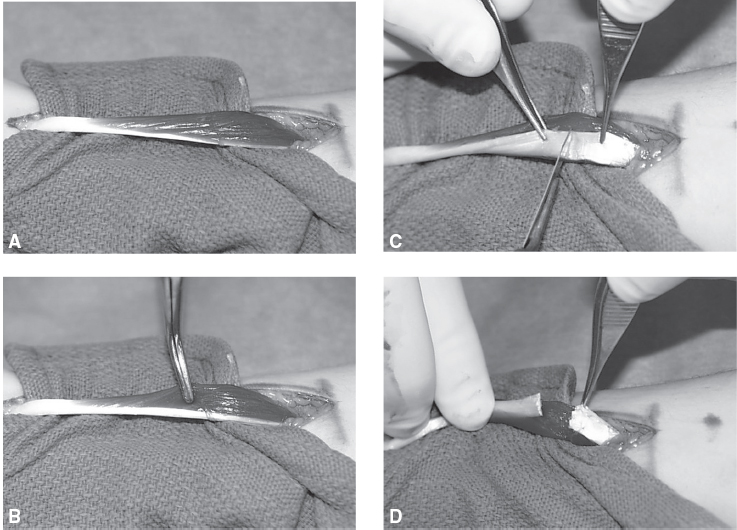
FIGURE 20.7 A fractional lengthening of a superficial finger flexor is shown. (A) A flexor digitorum superficialis is pictured at the time of surgery. The muscle belly is to the right and the tendon to the left of the figure. The myotendinous junction is the overlap region of muscle and tendon. (B) A surgical instrument is gently probing the muscle. (C) The tendon is being cut. There is extensive overlapping of the muscle in the region where the tendon is being cut. (D) After cutting the tendon, the muscle will elongate, effectively increasing the overall length of the muscle tendon unit.
Another surgical technique to decrease spasticity is a muscle slide or advancement. In this procedure, the entire origin of the muscle is advanced; it is done occasionally with the thenar muscles in the hand for a thumb contracture. By advancing the muscle origin, the distance across which a muscle works is shortened, in effect lengthening the muscle relative to its task. Lengthening techniques are directed at the tendon portion of the muscle and require long healthy tendon. Three such techniques that lengthen the tendon include a V to Y lengthening, a Z-lengthening, or a lengthening with multiple hemitenotomies. These additional four lengthening techniques differ from a fractional lengthening and are shown in Figure 20.9. Choosing one technique over another usually is based on specific anatomical constraints.
A difficulty of the Z-lengthening or a V–Y lengthening is that the surgeon is required to pick a new length for the tendon at the time of surgery. This is not an ideal situation because there is no way to make a precise decision intraoperatively and the surgeon is forced to guess the appropriate length. When a person with muscle spasticity undergoes surgery, he or she is under anesthesia, which reduces the active spasticity in the muscle, making it nearly impossible to judge the necessary lengthening. Once a new length is set with the Z-lengthening or V–Y lengthening, it is permanently established and cannot be changed. It takes about 3 months for the tendon to heal at its new established length during which time it must be protected or risk rupture. The lengthening with multiple hemitenotomies has similar disadvantages and is primarily used for the Achilles tendon. Achieving substantial lengthening with the V–Y lengthening, Z-lengthening, or the hemitenotomy lengthening technique is very difficult with small or weak tendons.
Tendon transfer is another important potential technique to treat spasticity. Tendon transfer is a technique that has shown great value in the treatment of peripheral nerve injuries, but has limited utility in the management of spasticity. Because of the unpredictable nature of spasticity, the tendon transfer may lead to overcorrection or undercorrection of a deformity and thus failure of the surgery. In this procedure, the surgeon detaches a tendon from one location and reattaches it to another. The intention of transfer is to redirect the force of the muscle. A deforming or destabilizing force can potentially be redirected into a useful, functional force. An example of a tendon transfer for spasticity is the split anterior tibialis tendon transfer for the equinovarus foot and ankle. In this transfer, the anterior tibialis tendon is split and the lateral half of the tendon is detached from the medial foot and is newly attached to the lateral foot (Figure 20.10). When performed properly, the anterior tibialis tendon becomes a neutral dorsiflexor of the ankle. This procedure can be successfully performed even with underlying spasticity being present.
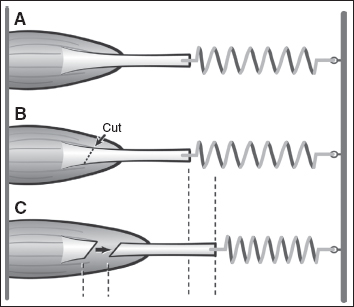
Figure 20.8 (A) The intrinsic spasticity of a muscle will determine the amount of lengthening that occurs with a fractional lengthening. (B) Once the tendon is cut, the amount of lengthening that occurs will be proportional to the tension in the muscle. (C) A muscle with little spasticity will exert only a small pull on the spring, and only a little lengthening will occur. For a muscle with a high degree of spasticity, once the tendon is cut, a stronger pull is exerted and a greater lengthening will occur.
In addition to the techniques of lengthening and transfers, other surgical techniques that can be potentially useful include neurectomy and joint arthrodesis. Neurectomy is the cutting of a nerve. This is irreversible and once a nerve is cut, no recovery can be expected. This can be valuable with a pure motor nerve such as the obturator nerve in the thigh or the ulnar motor nerve in the palm. When performing neurectomies, it is important to avoid interrupting significant sensory nerves. Sensory denervation increases the risk for skin breakdown in patients that may already have other risk factors for skin ulceration.
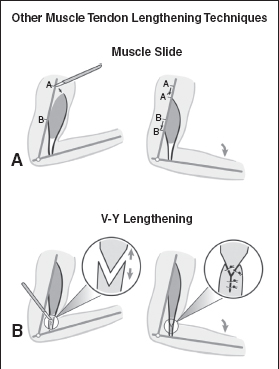
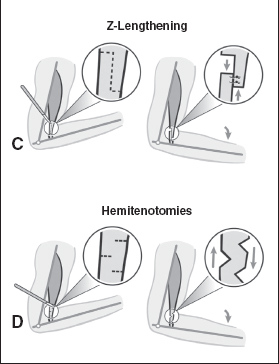
FIGURE 20.9 Four other lengthening techniques are depicted, including (A) muscle slide, (B) V-to-Y lengthening, (C) Z-lengthening, or (D) hemitenotomy lengthening. Choosing among a fractional lengthening and one of these other techniques is often dictated by anatomical constraints for each specific muscle.
Joint fusion procedures are performed occasionally when it is not possible or unnecessary to rebalance the joint. This is most commonly done at the wrist, ankle, or foot. For severe wrist or foot/ankle deformity, a fusion or arthrodesis can be done to permanently place the joint in one position for function. In the ankle, for example, a severe equinovarus deformity due to spasticity can be corrected with an ankle fusion placing the ankle in a permanent plantigrade (foot flat on the ground) position for sitting comfort, standing, or gait.
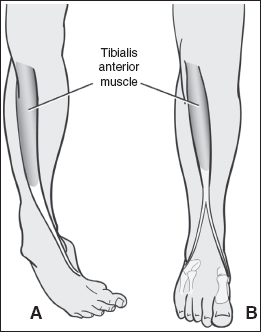
FIGURE 20.10 A split anterior tibialis tendon transfer is shown. The tendon transfer redirects the deforming force. In this example, (A) the anterior tibialis tendon causes a varus deformity of the foot before transfer. (B) By transferring the lateral half of the tendon into the lateral foot, balance is restored to the foot.
Planning Surgery for Elbow Spasticity
Planning is especially critical for any type of surgery, but especially when it involves spasticity. In this section, the author discusses the surgical planning process to treat elbow spasticity. For each of these elbow examples, the goal is the same: improved elbow extension. However, the goal is achieved in a very different manner for each of these examples. Lessons from these examples are applicable for any joint with spastic muscles producing disability. Reproducible surgical techniques are necessary and reasonable goals are always requisite for a successful outcome.
Information is necessary to plan a successful surgery. Knowledge regarding both the underlying structure (bones and joints) and the motors (muscles) is necessary to proceed. Once the skeleton has been evaluated, the muscles need to be considered, as a muscle-specific approach is necessary at the time of surgery. Physical examination alone is not adequate to understand individual muscle activity. The three main flexors of the elbow are the biceps brachii, brachialis, and brachioradialis and these may demonstrate varying degrees of spasticity and volitional control. Adequate agonist (flexors) and antagonistic (extensors) activity is necessary to have active function at the elbow. In addition to understanding the function of the agonist muscles, it is also critical to have a good understanding of the function of antagonist muscles at any joint including the elbow. If there is no volitional control or force generation of the elbow extensors, then it is unlikely that the patient will ever be able to actively extend the elbow even if the elbow flexors are lengthened and weakened. A surgery that lengthens the elbow flexors reduces their force generation, but if there is no antagonist extension force, the flexion deformity will recur with certainty. Balance at the joint can never be achieved if the extension force is zero until the flexion force also equals zero and then the elbow has no motors and this is not desirable either.
Instrumented, dynamic EMG provides critical muscle-specific information regarding activation, volitional control, and spasticity of each muscle (27–31). Although dynamic EMG can provide information regarding which muscles are spastic and to what extent there is volitional control, the dynamic EMG provides no information about how much force each spastic muscle may be contributing to a deformity. Estimates are therefore often necessary based on known muscle size and geometry to try and understand how much each spastic muscle is contributing to deformity at each joint under consideration. Once each muscle that can contribute to the deformity, both agonists and antagonists, is understood, then treatment can be planned. Each muscle can be left alone, lengthened, shortened, released, or transferred based on its function and contribution to the deformity.
A typical arm with a mild spastic elbow flexion contracture is pictured in Figure 20.11. The arm lacks about 30° of terminal extension. Four different dynamic elbow EMGs are pictured in Figures 20.12, 20.13, 20.14, and 20.15. Each of these dynamic EMGs was taken from four different elbows with spasticity. Any one of these four dynamic EMG studies could have been recorded from the elbow pictured in Figure 20.11 and there is no way to determine on clinical examination alone which one might have actually been the one recorded from this arm. Each of the elbows from which these four dynamic elbow EMGs was recorded will require a different surgical plan to optimize outcome. The information contained within the dynamic EMG allows the surgeon to plan a muscle-specific surgery to optimize outcome for the elbow.
The elbow dynamic EMG seen in Figure 20.12 shows passive elbow extension, or stretching of the elbow flexors. Of the three major elbow flexors, there is mild, spastic reactivity of all three elbow flexor muscles (biceps, brachialis, and brachioradialis) during passive stretching. A successful surgical plan for an elbow with this spasticity pattern will likely require lengthening of all three of the elbow flexors. A myotendinous lengthening should be performed for the biceps, brachialis, and the brachioradialis. For surgeons favoring a neurologic surgical approach to this problem, a selective partial denervation of the same three muscles may be considered.
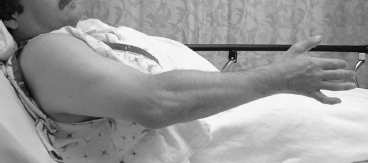
FIGURE 20.11 A patient with a spastic elbow flexion contracture lacks full active extension of the elbow. Physical examination alone does not allow predictable, accurate identification of the offending muscles. A dynamic EMG is necessary to study muscle activity during passive stretch and active movement to plan treatment, surgical or otherwise.
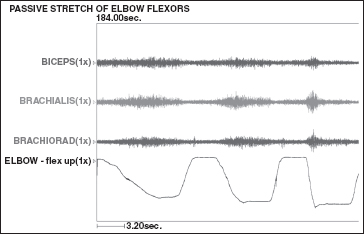
FIGURE 20.12 The recordings of a dynamic EMG of an elbow that lacks terminal extension due to elbow flexor spasticity. This recording shows the electrical activity in the three elbow flexors undergoing passive stretch. All three elbow flexors demonstrate spasticity. Courtesy of Nathaniel Mayer, MD.
The elbow dynamic EMG seen in Figure 20.13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






