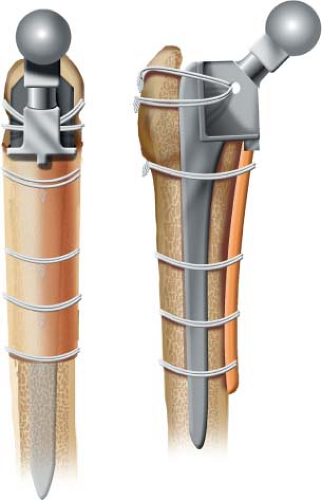
Strut Grafting of the Femur
Roger H. Emerson
Clinical Case
A 21-year-old female with polyarticular arthritis had bilateral total hip replacements in 1981. The components were cemented with a nonmodular femur, HD-2 design, with an all-polyethylene acetabular component. She also had bilateral total knee replacements in 1983. The right acetabulum was revised for polyethylene wear and aseptic loosening in 2000, at age 40, using a hemispheric bone ingrowth modular cup. At that time the femur was noted to be well fixed causing no pain but with some osteolytic areas in the calcar region. It was appreciated that the femur showed a cement column down to the distal isthmus, far beyond the tip of the stem, which would require a complex femoral revision. In the absence of symptoms the plan was to observe the right femur and leave the stem in place. By 2003 the patient had developed right thigh pain with weight-bearing and the radiographs showed a complete radiolucent line around the stem with some lateral debonding of the stem from the cement (Fig. 127.1). In October 2003 she underwent revision of the femur. The approach was transtrochanteric with a distal anterior window of the femur, to allow for the removal of cement.
Introduction
Bone allografting provides solutions to reconstructive problems involving significant bone loss, which is commonly encountered in the hip after fracture or failure of a joint prosthesis. A positive correlation exists between the amount of bone loss and the number of prior total joint surgeries. Restoring the bone stock of the femur is an important part of revision surgery (1,2,3). Experience has shown that successful allograft surgery, as with most orthopedic surgical techniques, requires special surgical knowledge and skills. The purpose of this chapter is to present the current status of onlay strut allografts used for reconstruction of the femoral component during revision total hip arthroplasty and after periprosthetic fractures.
The use of allograft bone to routinely restore major femoral bone stock after a failed prosthesis began in the early 1980s. Head et al. published a preliminary experience in 1987 of the first 14 cases, using a whole-bone allograft, with 15- to 30-month follow-up. Various fixation methods were used (4). The concept was to remove the damaged proximal femur and replace with an anatomically similar whole segment of allograft. This series has been followed and expanded, noting an increase in the complications with longer follow-up (5), but also with better overall clinical outcomes as a result of improved techniques. The most common serious complications were nonunion, dislocation, and deep infection. Tomford et al. (6) have shown that infection in revision total hip replacement is proportional to the complexity of the surgery rather than to the presence of allograft bone itself. With the goal of achieving a good bone reconstruction without extensive surgical exposure and removal of the least amount of host bone, placement of segmental pieces of allograft on the cortical femoral tube according to the anatomic or structure need became preferred over whole-bone grafts. These pieces of bone could be affixed easily with cerclage wires or cables. These became known as
cortical onlay strut grafts and found increasing use due to the simplicity and predictability of this grafting technique (Fig. 127.2). Allograft struts are especially adapted to the underlying biology of allograft healing due to the ease of rigid fixation to the underlying host bone and the broad area of contact with the host bone which promotes healing and remodeling.
cortical onlay strut grafts and found increasing use due to the simplicity and predictability of this grafting technique (Fig. 127.2). Allograft struts are especially adapted to the underlying biology of allograft healing due to the ease of rigid fixation to the underlying host bone and the broad area of contact with the host bone which promotes healing and remodeling.
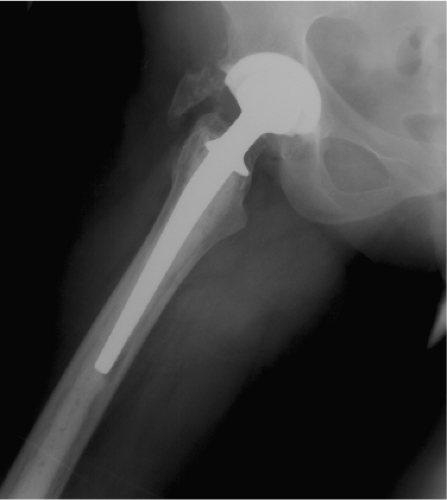 Figure 127.1. Preoperative lateral radiograph showing a loose cemented femoral component with a long cement mantle. |
Background
Several key breakthroughs in orthopedic knowledge have permitted modern allografting techniques to develop. These include a better knowledge of bone healing and repair, as well as knowledge of allograft bone immunogenicity. Allograft union represents a special type of normal bone healing and bone remodeling, where new factors come into play, especially immune mechanisms. The immune inflammatory response appears to diminish the amount of osteoinduction (7). It was not until Chase and Herndon (8) and Bonfiglio et al. (9) in 1955, as well as Curtis et al. (10) in 1959 established the diminished immunogenicity of frozen bone that modern allografting could go forward. Successful allografting requires bone union at the host–graft junction, followed by some degree of incorporation. Graft incorporation will not occur in the absence of union (11) Incorporation of bone with so-called “creeping substitution” initiated by osteoclasts, will lead to increased porosity of the graft which will diminish the strength of the bone which may be clinically significant if providing major implant support. Therefore, cortical grafts are strongest initially, but are weakened by the revascularizing repair process, about 40% from 6 weeks to 6 months in dog cortical autografts (11,12,13,14,15,16).
In a dog radius strut model, Malinin et al. (17) has shown by 24 weeks, although the grafts were distinguishable radiographically, they were visually blended with the host, and histologically had remodeled to new bone. Not only were the grafts remodeled, but also the host bone under the struts underwent similar remodeling. In a radiographic study of human struts by Emerson et al. (2), the bone grafts demonstrated the same sequence as in the dog model, and by 27 months the grafts appeared to be incorporated. Some resorption of these struts was evident, usually a rounding-off which occurred all along the edge of the bone plate. Also seen was hypertrophy of some of the bone struts where new bone was laid down on top of the graft at the proximal or distal ends. Further observation of these struts reveals that they ultimately take on the same appearance as the underlying host bone, responding to the same local mechanical environment as the host bone.
This critical role of mechanical rigidity in allograft union was appreciated by the early allograft pioneers. Parrish (18) noted that internal fixation was required for graft union. According to Goldberg et al. (7), the inflammatory response to allograft bone, especially fresh bone, abrogates the osteoinduction phenomenon by destroying or displacing the progenitor cells thereby preventing initial callus formation. Rigid internal fixation appeared to lessen the osteoinduction requirements for successful union to take place.
Modern studies have confirmed the decrease in immunogenicity of preserved bone and in addition have shown that different preservation methods produce different amounts of immune response. The amount and type of immune response also depends on the assay method as well as the animal model used (19) Freidlaender (20) compared deep freezing and freeze drying, using a microcytotoxicity assay for humoral and cell-mediated immunity in a rabbit model and found fresh cortical and corticocancellous allografts had significant levels of cell-mediated immunity. Deep frozen corticocancellous had decreased levels of immunity. Freeze-dried cortical showed no immunity, whereas freeze-dried corticocancellous produced some cell-mediated immunity in 2 of 5 cases. Therefore, of preserved grafts, freeze-dried cortex is the least antigenic, but both deep freezing and freeze drying are associated with a significant reduction in graft antigenicity when compared to fresh bone.
Indications
Improved bone stock will not always translate into a successful revision implant, but lack of sufficient bone has been known for many years to correlate with a diminished clinical outcome (1). Bone allografting is indicated whenever there is not sufficient host bone to revise the femur, or there is concern about the ability of the femur to support a revision component. The femoral component can be either cemented or cementless. Struts, since they are placed on the periosteal surface of the femur, have the advantage over bulk allografts of permitting a viable bone-implant interface with the possibility of bony ingrowth to the revision femoral component. Regional bone loss is ideal for strut reconstruction.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree