INTRODUCTION
Although stroke was once thought of as an incurable and static disease, numerous advances have been made over the past decade in the prevention, diagnosis, management, and rehabilitation of this disorder. In particular, the treatment and rehabilitation paradigm has improved significantly as a result of advances in acute interventions, risk reduction, medical devices, therapeutic modalities and exercise, robotics, diagnostic imaging techniques, and our overall understanding of the disease process itself. This resulted in a 35% reduction in the population-based death rate due to stroke between 1998 and 2008. Even with this progress, stroke remains a leading cause of death and disability in the world. Multidisciplinary stroke rehabilitation remains the primary treatment for post-stroke disability and should begin as soon as possible to optimize functional recovery and avoid potential complications and setbacks.
ACUTE DIAGNOSIS & MANAGEMENT OF STROKE
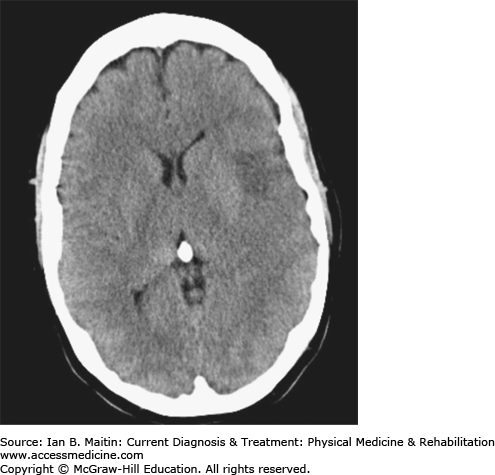
Stroke is a sudden-onset neurologic dysfunction resulting from focal disruption to the cerebrovascular system that requires rapid diagnosis and intervention. Acute neurologic symptoms are a medical emergency that justify immediate transport to the emergency department of an acute-care hospital for evaluation and treatment. It is vital to differentiate hemorrhagic from nonhemorrhagic (thrombotic or thromboembolic) strokes as soon as possible after onset of symptoms. Noncontrast computed tomography (CT) scan (Figure 14–1) is highly sensitive for acute bleeding and is commonly used for this purpose. Intravenous tissue plasminogen activator (tPA) should be considered for selected patients with acute thrombotic stroke within 3 hours of symptom onset, but other acute interventions can also be considered after that timeline.
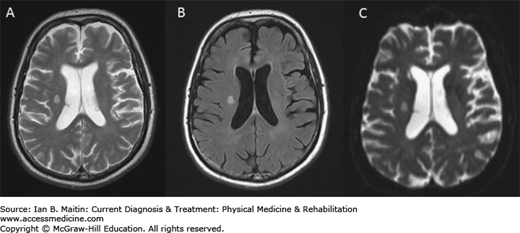
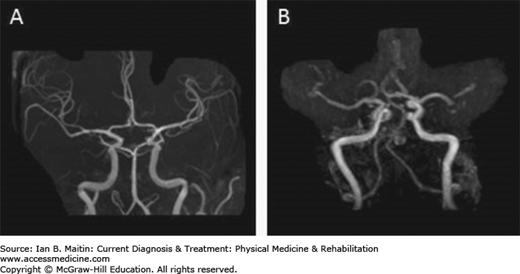
A thorough neurologic examination, combined with immediate neuroimaging study is indicated to evaluate acute neurologic symptoms suggestive of stroke. Magnetic resonance imaging (MRI) (Figure 14–2) is more sensitive for detecting posterior fossa lesions and acute ischemia within 24 hours of the stroke, especially using diffusion-weighted studies (Figure 14–2C). Patients with cardioembolic strokes caused by atrial fibrillation or associated with a proven embolic source in the heart and great vessels should be considered for anticoagulant therapy. Acute strokes that occur simultaneously in two areas of the brain subserved by different blood vessels are considered to be embolic until proven otherwise. Magnetic resonance angiography can help identify and characterize occlusion or stenosis of major cerebral vessels and the presence of cerebral aneurysms of moderate or large size. (Figure 14–3).
DEFINITIONS & GENERAL CONSIDERATIONS
Stroke, or cerebrovascular accident (CVA), is characterized by an acute onset of a neurologic deficit that persists for at least 24 hours and is the result of a focal lesion to the cerebrovascular system. A transient ischemic attack (TIA) is an acute-onset neurologic deficit that (apparently) resolves within 24 hours. However, approximately 17% of patients with TIAs suffer strokes within the next 3 months. In many patients with TIAs or other strokes with seemingly complete recovery, neuropsychological testing reveals long-lasting or permanent subclinical deficits; hence these events are associated with true damage, and all clinicians involved in post-stroke care should address risk factors aggressively to prevent future strokes.
Stroke is the fourth most common cause of death in the United States, behind heart disease, cancer, and chronic lower respiratory disease. Approximately 795,000 strokes occur per year in the United States, resulting in 135,000 deaths; approximately 610,000 of these strokes are initial incidents, and 185,000 are recurrent. Stroke is also the leading cause of long-term disability in the United States. Among elderly survivors 6 months post-stroke, approximately 50% have residual hemiparesis and 30% require assistance with activities of daily living or walking.
In 87% of patients, strokes are ischemic in origin, caused by occlusion of a blood vessel or other restriction of blood flow to a specific region of the brain. Thrombotic infarcts are the most common and are usually caused by occlusion of large cerebral arteries or their branches, most often as a result of atherosclerosis and progressive stenosis. Embolic infarcts are a subset of thrombotic infarcts and occur from distal passage of a thrombus or plaque from the heart, aortic arch, or proximal carotid arteries. Lacunar infarcts result from occlusion of small penetrating branches of cerebral arteries, often those that supply the basal ganglia, thalamus, internal capsule, and pons. They are often a consequence of atherosclerosis or degenerative changes in the arterial walls of these vessels secondary to longstanding hypertension or diabetes mellitus, or both. Up to half of single lacunar infarcts are clinically silent.
Hemorrhagic strokes (intracranial hemorrhages) account for 13% of all strokes. 3% of strokes are subarachnoid hemorrhages (SAHs), which usually result from rupture of intracranial arterial aneurysms. Intracerebral hemorrhages (ICHs), also called intraparenchymal hemorrhages, account for 10% of all strokes; they are often associated with hypertension and sometimes result from rupture of a vascular anomaly, such as an arteriovenous malformation. Intraventricular hemorrhage (IVH) can occur with any of these incidents.
PATHOGENESIS
Risk factors for stroke can be divided into nonmodifiable and modifiable factors. The modifiable risk factors encompass those associated with certain health habits and lifestyle-influenced diseases, reinforcing the central role of preventive efforts in reducing stroke morbidity and mortality. The major modifiable risk factors are high blood pressure, disorders of heart rhythm, smoking tobacco, dyslipidemias, physical inactivity, diabetes mellitus, sleep apnea, and chronic renal disease. Identification and management of these and other modifiable factors is crucial to the primary and secondary prevention of stroke and needs to be incorporated into the medical care of all stroke survivors undergoing rehabilitation.
Nonmodifiable risk factors are age, sex, race, geographic location, and heredity. The risk of stroke doubles every decade after 55 years of age. Females have a higher lifelong but lower age-adjusted stroke incidence than men (except over 85 years of age). Blacks have almost twice the risk and Hispanics have approximately 1.5 times the risk of stroke compared with non-Hispanic whites. There is a substantially higher incidence in the southeastern United States. Family history is important, as documented ischemic stroke in a parent before age 65 years is associated with a threefold increased risk of stroke. The presence of preexisting arterial disease in any form is also a nonmodifiable risk factor; stroke and coronary artery disease share many risk factors contributing to atherosclerosis; therefore, experiencing one increases the risk of developing the other.
is the most common modifiable risk factor for both ischemic and hemorrhagic stroke. In ischemic stroke victims, it likely accelerates the development of atherosclerosis and formation of atheromatous plaques. The plaque itself can result in stenosis and subsequent ischemia or provide a source of emboli, causing occlusion distal to the site of the lesion. More than 77% of patients who experience a first CVA have a blood pressure greater than 140/90 mm Hg. Diastolic hypertension may increase the risk of stroke up to seven fold. Hence, lowering blood pressure below 140/90 mm Hg (135/85 mm Hg for patients with added risk factors, such as diabetes and renal disease) results in a significant reduction in risk over time. Subjects with pressures less than 120/80 mm Hg have approximately half the lifetime risk of their hypertensive counterparts. However, especially during early rehabilitation started within several days of a stroke, blood pressure should be lowered cautiously, paying attention to neurologic status and performance in therapy, as rapid reduction risks clinical worsening and even enlargement of the ischemic stroke. Vasomotor instability can occur immediately following a stroke; the accompanying postural hypotension adds to that risk and should be vigilantly avoided.
Other important risk factors to address during rehabilitation, including diabetes mellitus, hyperlipidemia, and cigarette smoking, have been implicated in accelerated atherosclerosis. Smokers have two- to fourfold increased risk of CVA, and cessation has been shown to reduce that risk over time. The presence of diabetes mellitus nearly triples the risk of stroke; however, no conclusive evidence links tight blood glucose control to a decreased risk of stroke. Hyperlipidemia increases the risk of both heart disease and stroke. Treatment with HMG-CoA reductase inhibitors (statins) can reduce this risk in individuals with coronary artery disease and diabetes mellitus by up to 25%. An elevated high-density lipoprotein level is also protective. Underlying contributing factors to diabetes and hyperlipidemia, such as obesity and inactivity, themselves risk factors, should also be addressed. A creatinine level greater than 1.5 mg/dL has been associated with an elevated risk, likely due to accelerated atherosclerosis.
Antiplatelet therapy is advocated for primary and secondary prevention of stroke in patients with known atherosclerotic disease or a history of noncardioembolic ischemic stroke or TIA. Low-dose aspirin (81–325 mg daily) or clopidogrel is usually prescribed for primary and secondary prevention. Inpatients who suffer strokes while taking aspirin alone, secondary prevention with clopidogrel or the combination of aspirin and extended-release dipyridamole is usually initiated. Combining aspirin and clopidogrel may reduce the risk of further strokes but may also increase hemorrhage risk and is not routinely recommended unless the patient has other indications (ie, acute coronary syndrome or coronary stents). Cilostazol is used (off label) for secondary stroke prevention and has proven efficacy in patients of Asian origin, while ticagrelor is used to prevent thrombotic events after acute coronary syndromes.
Atherosclerosis often affects the carotid artery, especially near the carotid bulb. This and other areas may be subject to shear stresses from turbulent flow, resulting in a predilection to develop stenosis. A carotid bruit during physical examination may indicate carotid stenosis, although it does not necessarily correlate with severity. Studies have shown that symptomatic carotid stenosis (> 70%) has been associated with a substantial increased risk of ipsilateral and contralateral stroke and warrants referral for carotid endarterectomy. Although the risk of acute perioperative stroke or death in patients who undergo the procedure is 1.9% higher than in patients receiving medical therapy, the absolute risk reduction of endarterectomy over the next 5 years is 5.9% (55% relative risk reduction). Less-invasive endovascular angioplasty and stenting can also be considered, but this option remains controversial.
A variety of disorders can affect circulation to the brain. Autoimmune inflammatory vasculopathies produce changes in the vessel walls that may stimulate platelet adhesion and aggregation, resulting in thrombosis and distal embolism. It is vital that these vasculopathies be diagnosed and treated aggressively to prevent further strokes. With the exception of giant cell arteritis (temporal arteritis), these disorders usually affect small to medium-sized cerebral vessels.
Carotid and vertebrobasilar artery dissections can be post-traumatic or spontaneous and may lead to thrombus formation, arterial occlusion by narrowing of the vessel lumen, and embolization. Dissections should be treated emergently with therapeutic parenteral anticoagulation to prevent clot propagation and embolization distally. Migraines with aura (classic migraines) increase the risk of thrombotic stroke; sometimes the stroke can occur in the same vascular territory that produced transient neurologic symptoms during previous migraine attacks. Often, the role of migraine is questioned if there are other coexisting risk factors, especially smoking or estrogen use. Venous sinus thrombosis is often associated with otitis or sinusitis or a hypercoagulable state; anticoagulation is recommended.
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common hereditary stroke disorder and is thought to be caused by a mutation of the Notch 3 gene on chromosome 19. It is characterized by a progressive degeneration of the cerebral and extracerebral vascular smooth muscle cells leading to migraine headaches, TIAs, and strokes; the latter usually occur between 40 and 50 years of age. Fibromuscular dysplasia is usually an autosomal-dominant disorder characterized by fibrous changes to arterial structures, which may predispose children and young adults to thrombus formation and embolic strokes. Moyamoya disease is sometimes inherited as an autosomal-recessive disorder but also occurs in patients with severe atherosclerosis. It is characterized by progressive stenosis of the distal carotid arteries along with the adjacent middle cerebral artery branches and the presence of a fine network of collateral vessels at the base of the brain. This leads to the development of multiple arteriovenous malformations that can steal blood from surrounding tissue leading to infarction, or rupture and bleeding.
Sickle cell anemia, hypercoagulable states, thrombocytosis, polycythemia, and leukemias can all predispose a patient to cerebral ischemia. Sickle cell anemia typically affects the internal carotid artery or proximal portion of the middle cerebral or anterior cerebral artery. Hypercoagulable conditions include hereditary coagulopathies (factor V Leiden gene, protein C or S deficiency, and others) and acquired conditions, such as oral contraceptive use, cancer and the postpartum, post-traumatic or postsurgical state. Oral contraceptives in even low doses are associated with higher risk of stroke; additional risk factors such as smoking or migraine headaches, magnify this risk enormously.
Tobacco use is a major risk factor associated with ischemic stroke because of its role in accelerating atherosclerosis. Alcohol in moderation (less than 2 drinks per day) has been shown to cut the risk of stroke in half, whereas excess alcohol intake (greater than 5 drinks per day) can triple the risk of stroke. Insufflation of cocaine hydrochloride or smoking alkaloid (“crack”) cocaine can cause vasospasm resulting in an ischemic stroke. Intravenous abuse of heroin or other drugs predisposes individuals to infective endocarditis and consequent embolic strokes. Amphetamines, ephedrine, and phenylpropanolamine are stimulants that can raise blood pressure precipitously; these drugs are more commonly associated with ICH but may also cause vasospasm and cerebral ischemia.
Sleep apnea, physical inactivity, and pregnancy are also associated with an increased incidence of stroke. Sleep apnea has been shown to increase the risk two- to fourfold, depending on severity. Physical inactivity is linked to multiple other risk factors for stroke but may itself be independently associated with stroke. More ischemic strokes occur in the winter months when people are less active, and moderate to vigorous intensity exercise is associated with a lower incidence of stroke. Pregnancy without the presence of preeclampsia is a risk factor for both ischemic and hemorrhagic strokes, especially in blacks and older women. Postpartum strokes are associated with cardiovascular disease, but not strokes that occur before delivery. Stroke during pregnancy usually necessitates termination of the pregnancy to enable aggressive treatment of the mother.
Patients with atrial fibrillation, especially those with valvular disease, have a markedly increased chance of suffering an embolic stroke each year. Most will require lifetime therapeutic anticoagulation, which often is started or continued during acute rehabilitation. Warfarin (INR goal of 2–3) has been shown to decrease the risk of stroke by 61% in patients with atrial fibrillation. Dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, direct factor Xa inhibitors, also reduce the risk of stroke from nonvalvular atrial fibrillation. When anticoagulation is contraindicated, antiplatelet agents are recommended even though they are inferior to warfarin for embolic prophylaxis. Dilated cardiomyopathy and myocardial infarction are associated with intracardiac mural thrombus formation leading to cerebral embolism. The risk of development of mural thrombus correlates with the size of the myocardial infarct; hence patients with large cardiac lesions may require anticoagulation. Structural heart diseases, particularly mitral valve stenosis and prolapse, have also been associated with increased risk but usually do not warrant prophylactic anticoagulation. Mechanical prosthetic heart valves, however, are highly prone to platelet adhesion and thrombus formation and therefore necessitate long-term anticoagulation therapy with warfarin (INR goal of 2.5–3.5). Left ventricular assistive device implantation is also associated with elevated stroke risk, which may be further increased by postoperative infections.
Theoretically, atrial septal defect and patent foramen ovale allow for blood flow between the right and left sides of the heart and may allow for passage of embolic material from the venous circulation in the extremities to the brain. Thus, a deep venous thrombosis in the leg may give rise to a “paradoxical” embolic CVA. Conditions that increase right heart pressures, such as multiple pulmonary emboli and severe obstructive sleep apnea, can increase the flow of blood through the septal opening. Intravascular repair of these defects is sometimes considered in patients with cryptogenic stroke who are found to have a patent foramen ovale; however, repair is not proven to reduce future stroke risk.
Both infective and marantic endocarditis can lead to formation of vegetations on the valve leaflets, which may also lead to thromboembolic events. Infective endocarditis is usually seen in intravenous drug users and patients with valvular disease or prosthetic valves and can be bacterial or fungal in etiology. Prolonged antibiotic treatment is critical. Marantic endocarditis is typically seen in cancer patients, most often those with adenocarcinomas of the lung or gastrointestinal tract. Anticoagulation should be strongly considered in these patients.
ICH is usually associated with hypertension. Common affected areas include the basal ganglia, thalamus, pons, cerebellum, and occipital lobes. In older patients, particularly when hemorrhage occurs in other areas, the most common cause is thought to be angiodysplasia from cerebral amyloid angiopathy. These individuals often have lobar hemorrhages at multiple sites.
Other causes of ICH are wide and varied. Heavy alcohol intake raises the risk of ICH in selected populations. Vascular malformations such as cerebral angiomas and aneurysms may also cause other symptoms, such as seizures and headaches, prior to any actual bleeding. The initial presentation of some ischemic strokes, especially embolic, may be accompanied by hemorrhagic transformation, which may lead to clinical worsening. Patients with coagulation deficits, such as hemophilia and von Willebrand’s disease, and those receiving anticoagulation therapy are at an increased risk for developing spontaneous ICH. Intravenous tissue plasminogen activator (tPA) administered acutely within 4.5 hours of stroke onset is associated with a 7% risk of symptomatic ICH. Vascular malformations and primary or metastatic brain tumors also predispose patients to ICH. In general, all ICH of uncertain etiology warrants follow-up contrast imaging to rule out tumors or other mass lesions obscured by acute blood on initial evaluation.
SAHs usually result from a ruptured saccular (berry) aneurysm or arteriovenous malformation, but can also be due to trauma. The classic presenting symptom is sudden onset of an unusually severe headache followed frequently by vomiting, neck stiffness, and collapse with loss of consciousness. Since bleeding occurs mainly in the subarachnoid space, prominent focal neurologic deficits are less common on neurologic examination. One exception is an aneurysm of the posterior communicating artery or middle cerebral artery, which may result in ipsilateral cranial nerve III palsy from local compression. Associated vasospasm can cause focal infarctions, which greatly complicate acute symptoms and eventual disability. Intracranial blood can be very irritating to surrounding neural tissues and is associated with cerebral edema. Symptoms vary considerably, depending on the amount of cerebral edema surrounding the hemorrhage, and the capacity of the brain to accommodate the edema.
CLINICAL FINDINGS
Ischemic strokes due to thrombosis or emboli often produce focal neurologic deficits that correlate with the region of the brain supplied by the underlying vascular lesion. A thorough neurologic examination can often localize the lesion. However, physical examination alone cannot differentiate between hemorrhagic and thrombotic stroke; thus, imaging studies are needed acutely. The vascular territories and usual neurologic examination findings for major large vessel cerebral infarctions are shown in Table 14–1. Major brainstem stroke syndromes are listed in Table 14–2. Lacunar infarcts in the brain result from occlusion of small penetrating branches of the major cerebral arteries and are frequently clinically silent and incidental findings on brain imaging. Although neurologic deficits can vary widely, five distinctive lacunar syndromes that occur frequently have been identified (Table 14–3). ICH is more variable in presentation owing to variations in size and location, particularly the lobar hemorrhages. Pontine, cerebellar, and deep cerebral (putamen and thalamic) hemorrhages often causes omnolence or coma because of their location proximal to the reticular activating system. Hemorrhages in the basal ganglia cause hemiparesis and, if sizable or surrounded by edema, can cause hemianopia, inattention, or aphasia. Thalamic hemorrhages usually cause hemisensory deficits. Brainstem hemorrhages can cause hemiparesis or quadriparesis, severe dysphagia, dysarthria, and gaze deficits. Cerebellar hemorrhages can cause ataxia of the trunk or limbs and vertigo.
| Vascular Territory and Possible Areas Damaged | Possible Neurologic Deficits |
|---|---|
| Anterior cerebral artery (medial aspect of frontal or parietal lobes) | Contralateral hemiparesis of the leg Contralateral hemisensory deficits in leg Impaired bladder inhibition Personality changes Ideomotor apraxia Abulia Gegenhalten rigidity, alien arm or hand |
| Dominant hemisphere | Transcortical motor or mixed aphasia |
| Severe damage or bilateral lesions | Akinetic mutism Paraplegia |
| Superior division of MCA (lateral aspect of frontal or frontoparietal areas) | Contralateral hemiparesis of the face, hand, and arm > leg Contralateral hemisensory deficits in the face, hand, and arm > leg |
| Dominant hemisphere | Expressive (Broca’s) aphasia |
| Nondominant hemisphere | Hemineglect, constructional and dressing apraxia |
| Inferior division of MCA (lateral aspect of posterior parietal or temporoparietal areas) | Contralateral homonymous hemianopsia Homonymous quadrantanopsia Contralateral graphesthesia and stereognosis Anosognosia Dressing and constructional apraxia |
| Dominant hemisphere | Receptive (Wernicke’s) aphasia |
| Nondominant hemisphere | Left visual neglect |
| Bilateral lesions | Simultagnosia |
| Posterior cerebral artery (tempero-occipital or occipital areas) | Contralateral homonymous hemianopsia Vertical gaze palsy Oculomotor nerve palsy Ataxia Internuclear ophthalmoplegia Anomic or transcortical receptive aphasia Alexia without agraphia Visual agnosia Prosopagnosia |
| Bilateral lesions | Cortical blindness, memory impairment Simultagnosia |
| Basilar artery branches (brainstem or cerebellum) | Unilateral or bilateral abducens nerve palsy Impaired horizontal eye movements Ipsilateral hemiparesis or quadriparesis Ipsilateral hemisensory deficits Balance disturbance Contralateral ataxia Dysarthria Dysphagia |
| Bilateral basilar artery branches (Ventral portion of pons is infarcted and tegmentum spared) | Locked-in syndrome: Only eye opening, vertical eye movements, pain, and temperature sensation spared Quadriplegia Mutism |
| Vascular Territory | Neurologic Deficits |
|---|---|
| Weber syndrome (occlusion of interpeduncular branches of posterior cerebral or posterior choroidal artery to base of medial midbrain) | Ipsilateral oculomotor palsy Contralateral hemiplegia |
| Benedikt syndrome (occlusion of interpeduncular branches of posterior cerebral or basilar artery to lateral midbrain) | Ipsilateral oculomotor nerve palsy and mydriasis Contralateral sensory loss Contralateral ataxia, tremor, chorea, and athetosis secondary to damage to red nucleus |
| Millard–Gubler syndrome (occlusion of circumferential branches of basilar artery to medial pons) | Ipsilateral abducens and facial nerve palsy Contralateral hemiplegia Contralateral sensory loss |
| Occlusion of superior cerebellar artery to lateral portion of rostral pons | Impaired optokinetic nystagmus Contralateral loss of vibration, position, pain, temperature, and tactile sensation |
| Occlusion of anterior inferior cerebellar artery to lateral portion of caudal pons | Ipsilateral facial weakness Abducens nerve palsy Contralateral hemiplegia Contralateral sensory loss |
| Lateral medullary syndrome or Wallenberg syndrome (most cases due to vertebral artery or posterior inferior cerebellar artery infarction) | Ipsilateral cerebellar ataxia Ipsilateral Horner syndrome (ptosis, anhydrosis, and miosis) Impaired pain and temperature sensation of ipsilateral face and contralateral body Vocal cord paralysis, dysphagia, dysarthria Vertigo, nausea, vomiting, and hiccups Nystagmus or diplopia, or both |
| Medial medullary syndrome | Ipsilateral hypoglossal nerve palsy Contralateral hemiplegia Contralateral sensory loss |
| Anatomic Location of Lesion | Neurologic Deficits |
|---|---|
| Posterior limb of internal capsule or ventral pons | Contralateral hemiparesis of face, arms, and legs |
| Ventrolateral thalamus | Contralateral hemisensory deficits with paresthesias |
| Ventral pons, internal capsule, or subcortical white matter | Contralateral hemiparesis and ataxia primarily of leg |
| Ventral pons or genu of internal capsule | Dysarthria, dysphagia, contralateral facial weakness, and clumsiness of contralateral hand |
| Genu or anterior limb of internal capsule | Contralateral hemiparesis with motor aphasia (if dominant side) |
In addition to a neurologic examination, physical examination should also include a comparison of left and right blood pressures and pulse strength, auscultation of the neck for carotid bruits, careful cardiac examination for arrhythmias or murmurs, and(especially in those with headaches) palpation of the temporal arteries. An ophthalmoscopic examination can also be useful, especially in patients with hemorrhagic strokes or diabetes mellitus. Visual confrontation examination of the diabetic stroke patient should be performed with each eye separately.
DIFFERENTIAL DIAGNOSIS
TREATMENT
Numerous advances have recently been made in the prevention, treatment, and rehabilitation of stroke. This has resulted in a significant reduction in the population-based death rate over the past decade. Once thought of as an incurable and static disease, stroke is now viewed as a treatable acute condition. In some dramatic cases, intervention with fibrinolytic therapy or mechanical thrombectomy has led to complete resolution of symptoms in days with apparent full recovery. Early interdisciplinary rehabilitation remains the primary recommended treatment for post-stroke disability.
Rehabilitation is a holistic process involving multiple health care professionals that should begin almost immediately after stroke to maximize the survivor’s potential for functional recovery. Even patients intubated in the intensive care unit should receive range-of-motion exercises and frequent repositioning in bed to prevent future complications such as decubitus ulcers and contractures that can further impede progress.
Some of the initial recovery from stroke is presumed to result from resolution of the penumbra or cerebral edema. The penumbra is the margin of reversible ischemia surrounding the infarcted core of the CVA. Edema around the lesion can impair brain function, and resolution of this response may lead to significant recovery. Following ICH and SAH, resorption of blood and resolution of associated cerebral edema are likely responsible for early clinical improvement. The axons of partially spared pathways may reinnervate or sprout over several months or longer, contributing to later recovery.
It is now widely accepted that with stimulation the brain has a great potential for neuroplasticity-induced changes after stroke. A large body of evidence has shown that the brain can often change and continue to regain function months or years after the initial event. These changes are associated with alterations in neurotransmitters and their synaptic receptors, and much research has been directed toward determining whether pharmacologic modulation of these neurotransmitters can facilitate changes. Medications that have been studied include those that affect noradrenergic, dopaminergic, cholinergic, and serotonergic receptors. Recently published studies suggest that serotonin reuptake inhibitors (SSRIs), widely used for prophylaxis or treatment of post-stroke depression, may facilitate motor recovery. The evidence supporting the benefits of the other classes of medication has been weaker.
The basic goal of intensive post-stroke rehabilitation is to maximize independence, and the vast majority of stroke survivors will respond to intensive interdisciplinary rehabilitation by making gains. However, some rehabilitation hospitals and units restrict access to such intensive treatment for stroke patients who lack realistic goals. For example, a patient who lives alone and suffers a severe right brain stroke with residual perceptual and motor weakness and likely permanent need for assistance may have the unrealistic goal of returning home independently. Such a patient may be rejected for admission to some units, especially if resources for placement (in custodial nursing facilities) are lacking. It should be understood that such a patient would likely make significant gains in intensive rehabilitation and ultimately require substantially less assistance after discharge than if sent directly from an acute hospital to a nursing facility. Such a reduction in the amount (and cost) of care may justify admission to intensive rehabilitation (but the patient would have to agree, at some point, to change his or her discharge goal).
Generally, acute stroke patients are evaluated in the acute care hospital by a physical therapist or rehabilitation physician, who determines the level of mobility impairment and the ability to tolerate therapy. Self-care deficits are identified by nursing and, when needed, occupational therapy providers. Occupational therapy participation is particularly important in the acute hospital when evaluating the patient for whom discharge home is a consideration. Assessment by a speech therapist is important acutely to determine whether dysphagia is severe enough to warrant nothing-by-mouth (NPO) status, a modified consistency diet, or enteral feeding.
The term subacute care has been applied to a broad range of medical and rehabilitative services and settings that provide care to patients following acute management in a hospital setting. Medicare has five levels of skilled nursing facility rehabilitation. The two most intensive of these are considered subacute level of care, providing 1.5–2.5 hours of therapy per day, 5 days per week. For Medicare to pay for skilled nursing facility rehabilitation, the patient must have had a 3-day inpatient hospitalization in the past 30 days (observation status is not included)and require skilled nursing or therapy services.
A long-term acute care hospital is a hospital that specializes in the medical treatment (and sometimes rehabilitation) of medically complex patients who require an extended stay in a hospital setting; often the medical focus involves weaning patients from ventilators. Some stroke patients referred for acute intensive rehabilitation who have potentially unstable medical problems should be considered for treatment in a long-term acute care hospital; once the complex medical problems stabilize and the patient demonstrates consistent tolerance of therapies, transfer to an acute intensive rehabilitation unit may be appropriate.
Studies have shown that for Medicare fee-for-service beneficiaries needing rehabilitation after stroke, comprehensive treatment in intensive rehabilitation hospitals leads to, on average, greater functional improvement and more frequent community discharge than patients receiving subacute rehabilitation services at skilled nursing facilities.
DVT occurs in the hemiparetic lower extremity 60–75% of the time. In the vast majority of stroke patients, DVT is not painful; only 3–5% have clinical symptoms (limb swelling, fever, pain, or a combination). DVT risk factors include advanced age, severe limb paresis, immobility, dehydration, previous venous thrombosis, and the presence of neoplasm. Pulmonary embolism occurs in 1–2% of stroke patients. Half of patients with symptomatic pulmonary embolism die at presentation; thus, it is now a standard of medical practice that thromboembolic prophylaxis be initiated or otherwise addressed for all acute stroke patients and continued during intensive rehabilitation.
DVT is diagnosed by ultrasonography, impedance plethysmography, contrast venography, or d-dimer assays. Currently, diagnosis is usually made by duplex ultrasonography, which is more sensitive and specific in the thigh than in the calf. For pulmonary embolism, pulmonary angiography is most sensitive and specific, but radionuclide ventilation perfusion scan is also used. 90% of pulmonary emboli are associated with proximal lower limb DVT. Prophylaxis includes subcutaneous heparin given every 8 hours, low-molecular-weight heparin, or intermittent pneumatic compression if patients have contraindications to prophylactic anticoagulation. If pneumatic compression devices are used it is essential to monitor the skin. It is generally safe to combine antiplatelet therapy with prophylactic anticoagulation.
The goal of therapeutic treatment of acute DVT is to prevent extension and propagation of the clot. If anticoagulation is contraindicated, an inferior vena cava filter should be considered. These filters have an acceptable safety profile in stroke patients. If possible, therapeutic anticoagulation should be started later, to limit clot propagation. If the patient lacks other procoagulant risk factors and is likely to regain ambulatory ability, a retrievable interior vena caval filter should be inserted.
Screening all patients for DVT at the time of admission to the rehabilitation unit is controversial, largely because most clots diagnosed will be in the calf and may not warrant any therapeutic treatment. Screening is more strongly indicated if the patient has had no prophylaxis or a gap in prophylaxis prior to admission. A calf clot should be followed with a repeat study within 1 week, to determine if the clot is propagating; if so, therapeutic anticoagulation is indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree