Fig. 6.1
Oblique lumbar radiographs demonstrating spondylolysis (arrows). (a) The pars interarticularis is the “isthmus” located between the superior and inferior articular facets and between the lamina and pedicle. (b) This corresponds with the neck of the “Scotty dog” seen on oblique radiographs
The mechanism for a pars injury involves repetitive extension or rotation of the lumbar spine, which causes shearing forces to develop between the inferior articular process of the level above and the superior articular process of the level below. Capener first described this “bony pincer” mechanism in 1931 [41, 43], which is consistent with the higher prevalence of spondylolysis seen in athletes whose discipline includes repeated lumbar extension [11]. Investigators have subsequently studied specific lumbosacral factors that predispose to an injury in this region, finding that greater anterior inclination angles of the lumbosacral endplates and increased degrees of lumbar lordosis correlate both with increased rates of pars fractures at L5 compared to age-matched controls and decreased rates of union following conservative management [17, 44]. Chung et al. also found that a smaller inter-facet distance from L4 to S1 and a greater lordosis at L5 to S1 are associated with L5 spondylolysis in young patients [45].
Congeni has described three subpopulations that account for the variable biomechanical factors that can predispose to injury [46, 47]. Type I is a hyperlordotic female athlete with increased range of motion and flexibility, such as a dancer or gymnast. Type II is a muscular male athlete with decreased hamstring and erector spinae flexibility who is undergoing a rapid growth spurt, such as football players and weightlifters. Type III is an athlete new to a sport or activity and undergoing a newly increased amount of training, often in conjunction with poor core strength and trunk flexibility. Understanding the factors that predispose to injury is important and can potentially influence the success of a rehabilitation treatment program.
History and Physical Exam
There have been few studies that objectively assess the clinical presentation of symptomatic spondylolysis, and the vast majority of patients who have radiographic evidence of a pars defect developed the condition without symptoms [4, 41]. Many authors describe the typical presentation as focal low back pain, with occasional radiation into the buttock or proximal lower limb [31, 38, 48–51]. Onset may be insidious or after an acute injury, and athletes with low-grade symptoms may experience more severe symptoms after a period of heavy exertional stress [52, 53].
Studies to determine the diagnostic accuracy of physical examination maneuvers are also scarce. Findings frequently described as characteristic of spondylolysis include exaggerated lumbar hyperlordosis, ipsilateral paraspinal muscle spasm, tight hamstrings, pain with extension, and tenderness to palpation over the site of the pars lesion [11, 47, 49, 51, 53, 54]. Neurologic examination in isolated spondylolysis should be normal, and other diagnoses should be considered when patients present with radicular symptoms.
Many clinicians have cited the one-legged hyperextension test as pathognomonic for spondylolysis [49, 50, 53, 54]. This maneuver is positive when the athlete leans backward while standing on one leg if the motion reproduces his or her concordant pain (Fig. 6.2). Masci et al. studied the diagnostic accuracy of this test in young, active subjects with low back pain, but found no association of a positive one-legged hyperextension test with the presence of active spondylolysis diagnosed by single-photon-emission computed tomography (SPECT) (followed by CT, if positive) and MRI [29]. Thirty-nine of the 71 subjects (54.9 %) were found to have spondylolysis. Sensitivity and specificity for pars lesions ranged from 50 to 55 % and 46 to 68 %, respectively, depending on side. Moreover, the positive likelihood ratios ranged from 1.01 to 1.54 and the negative likelihood ratios ranged from 0.98 to 1.01, suggesting that the test has limited ability to alter pretest probability. Given the high prevalence of spondylolysis in adolescent athletes presenting with low back pain, it is imperative for clinicians to maintain a high index of suspicion for this condition any time a young athlete presents with low back pain.
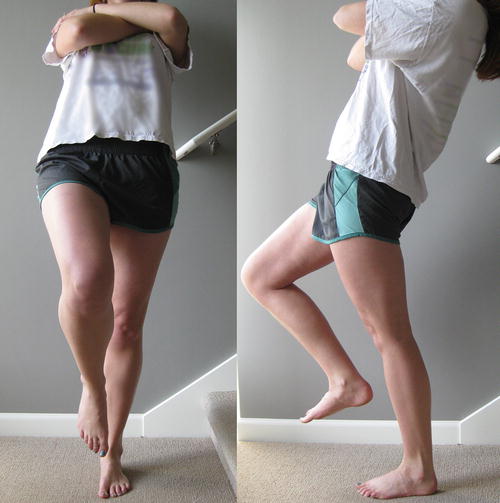

Fig. 6.2
One-legged hyperextension test. The examiner asks the athlete to raise one leg and lean backward. It is positive if it reproduces the patient’s concordant symptoms of low back pain [29]
Diagnostic Imaging
Establishing evidence of a symptomatic spondylolysis poses several diagnostic challenges. Many radiologic findings are common in asymptomatic individuals, underscoring the importance of correlating an athlete’s symptoms with positive imaging results. The clinician must consider not only the sensitivity and specificity of any test ordered but also the risk of cumulative radiation exposure. There have been no direct comparisons of clinical outcomes and treatment based on diagnostic imaging choice [2]; thus, a thoughtful approach is necessary to optimize treatment and return-to-play decisions based on diagnosis and stage of injury.
Radiographs
The oblique course of the pars interarticularis relative to the vertebral column limits the sensitivity of routine radiographs in visualizing an abnormality of this region (Fig. 6.3). A defect is described as a lucency or discontinuity involving the collar of the “Scotty dog” seen on lateral oblique radiographs (see Fig. 6.1). Diagnostic accuracy depends on both the orientation of the fracture line and the degree of cortical disruption [55, 56]. Early studies found that clinicians were not able to view 12–20 % of fractures when using anteroposterior (AP) and lateral views alone. This led many to suggest that oblique views were necessary to adequately visualize an isthmic defect [3, 57, 58].
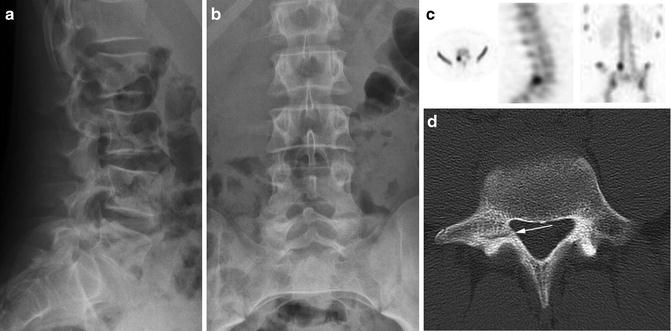

Fig. 6.3
Images taken from a 14-year-old young male soccer player diagnosed with active spondylolysis after 3 months of progressive low back pain. Although initial imaging demonstrated normal (a) AP and (b) lateral radiographs, the diagnosis was later established by (c) SPECT (axial, sagittal, and coronal views shown) and (d) CT (the arrow points to an early pars defect)
However, more recent work has challenged the diagnostic value of oblique radiographs, and in 2013, Beck et al. demonstrated no statistical difference between the sensitivity of 2-view and 4-view studies in detecting spondylolysis (Table 6.1) [59]. Considering the fact that only one-third of pars lesions are aligned within 15° of the lateral oblique plane [56], this newer data is not surprising. The additional cost and risk of ionizing radiation exposure involved in obtaining oblique views (increased by 75 %, see Table 6.1) likely overshadow any routine diagnostic benefit [59].
Table 6.1
Radiation dose and diagnostic value of 2-view or 4-view lumbar radiographs for spondylolysis [59]
Modality | Radiation dose (mSv) | Sensitivity | Specificity |
|---|---|---|---|
2 view: AP/lateral | 0.72 | 0.59 | 0.96 |
4 view: AP/lateral, bilateral oblique | 1.26 | 0.53 | 0.94 |
Bone Scintigraphy
As noted before, pars defects are common. They have an estimated prevalence of 4–6 % within the general population by age 18 and 8–26 % of elite adolescent athletes [4, 11, 60]. Thus, the sports physician must discern not only whether a lesion is present but also whether it correlates with an athlete’s symptoms. Treating an inactive nonunion could lead to delays in definitive treatment and eventual return to sports. Nuclear medicine studies, such as bone scans and SPECT, are sensitive in detecting the metabolic bone activity associated with a symptomatic lesion. They can detect an early stress reaction and offer prognostic information about whether or not a defect is likely to respond to treatment [61].
The three-dimensional nature of SPECT favors its use over simple bone scans by helping to localize an abnormality to the posterior elements of the spine. Unfortunately, although SPECT can detect early lesions prior to evidence of an abnormality on CT or MRI [29, 62], it exposes the athlete to ionizing radiation and lacks the specificity to justify its use in isolation. Thus, although SPECT represents the most sensitive diagnostic standard in detecting a symptomatic spondylolysis, other imaging modalities are needed to grade the injury and differentiate it from other less common diagnoses, such as tumor or infection [63].
Computed Tomography (CT) and Staging
For symptomatic lesions identified on SPECT, fine-cut CT (1 mm slices) scans provide the necessary osseous and cortical detail to accurately stage spondylolysis [63]. Generally, because of added radiation (see Table 6.2) [64], attention is turned to only 1–2 levels based on metabolic activity seen on prior scintographic analysis. Unfortunately, CT scans are not as sensitive as SPECT, and up to 20 % of pars abnormalities seen on SPECT are not demonstrated on thin slice CT [62]. These lesions likely represent early stress reactions [63], which are important to detect as they warrant special treatment considerations to facilitate bony healing and limit progression to a true fracture.
Morita et al. first described a classification system of three categories for pars lesions based on CT characteristics: early, progressive, and terminal [65] (Fig. 6.4). These categories accounted for the severity of injury, degree of cortical discontinuity, as well as the chronicity of the lesion. “Early” lesions involve a small fissure or a hairline defect through the pars. “Progressive” pars defects are wider and associated with occasional small fragments. Sclerotic cortical changes characterize “terminal” lesions, which are also known as pseudoarthrosis.
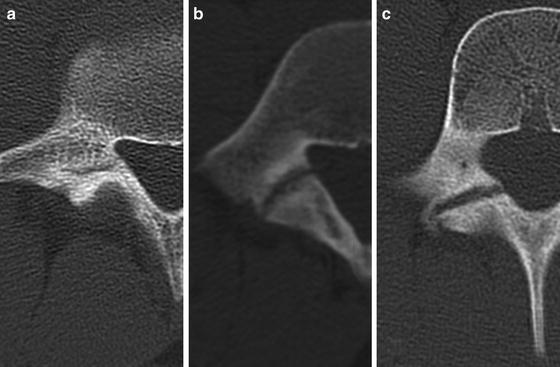

Fig. 6.4
CT demonstrating early, terminal, and progressive right-sided spondylolysis. (a) Early lesions involve a small fissure or hairline defect through the pars. (b) Progressive pars defects are wider and associated with occasional small fragments. (c) Sclerotic cortical changes characterize terminal lesions or pseudoarthrosis [65]
Grading the severity of a pars stress injury becomes particularly important when discussing prognosis or return to play, as early stress injuries heal more readily than progressive fractures and certainly more than terminal ones which rarely, if ever, heal [17, 66]. This was demonstrated by Fujii et al., who examined 239 pars defects and found evidence for bony union after treatment of 62 % of early stage lesions, compared to 8.7 % of progressive lesions and 0 % of terminal lesions [17]. Sairyo et al. later attempted to correlate both CT and MRI findings with clinical outcomes and found that in a study of 68 pars defects, early lesions (86 %) or progressive lesions with high T2 MRI signal changes involving the adjacent pedicle (60 %) were the most likely to heal [66]. Similar to previous findings, none of the terminal lesions developed evidence of bony union.
Magnetic Resonance Imaging (MRI)
Given concerns over radiation exposure from SPECT and CT (see Table 6.2), there will be increasing pressure to optimize and incorporate MRI protocols and techniques. Although cortical detail is sacrificed compared to CT, MRI is excellent at visualizing soft tissues when considering alternate diagnoses. MRI may play a role in staging spondylolysis, particularly when T1 and T2 changes suggest edema in the area of the pars or pedicle [66]. But not all bony defects are visible, and the sensitivity of MRI greatly depends on image slice thickness, spacing, and orientation.
One recent case series of 74 athletes found MRI missed the diagnosis in 64 % of patients who had been diagnosed by CT [67], but Campbell et al. were the first to directly compare MRI, CT, and SPECT [68]. Using SPECT with CT as the diagnostic standard, they established that although MRI was able to detect 39/40 (98 %) of the pars defects seen, it correctly graded only 29 (72 %) of the lesions. The greatest discrepancy involved earlier stage injuries, or those with the greatest potential for healing [17].
Unfortunately, the study by Campbell et al. utilized nonstandard image sequences (oblique sagittal images), limiting the ability to generalize the results. Studies of more standard sequences reveal that MRI likely only detects around 80 % of pars lesions seen on SPECT [29]. As work emerges examining the utility of stronger magnets (3 T) and improved imaging protocols, perhaps MRI will one day emerge as the new “gold standard” in diagnosing early stage lesions. But until these protocols are adopted as standard practice, the discerning clinician should exercise caution when interpreting data or basing treatment decisions solely on MRI findings.
Reasonable Imaging Algorithm
Standaert and Herring outlined a reasonable imaging algorithm based on a thorough review of the available literature (Fig. 6.5) [63]. This involves first obtaining limited plain radiographs (i.e., standing AP and lateral) to identify a spondylolisthesis or other gross bony abnormality. Assuming these findings do not change diagnostic considerations, a SPECT scan of the lumbar spine should be performed. If this identifies an area of increased metabolic activity in the pars interarticularis, then a thin-cut CT (1-mm axial sequences) can be performed through only the identified level(s) to confirm the diagnosis and to stage the lesion, thus helping to gauge prognosis. If the SPECT is negative, then other diagnoses should be considered. If symptoms are present for greater than 6 weeks, further evaluation with MRI is reasonable. At the end of treatment for a spondylolysis, follow-up standing lateral radiographs should be considered every 6–12 months in athletes with bilateral defects or if a listhesis is already present to evaluate for progressive slip until the patient reaches skeletal maturity.
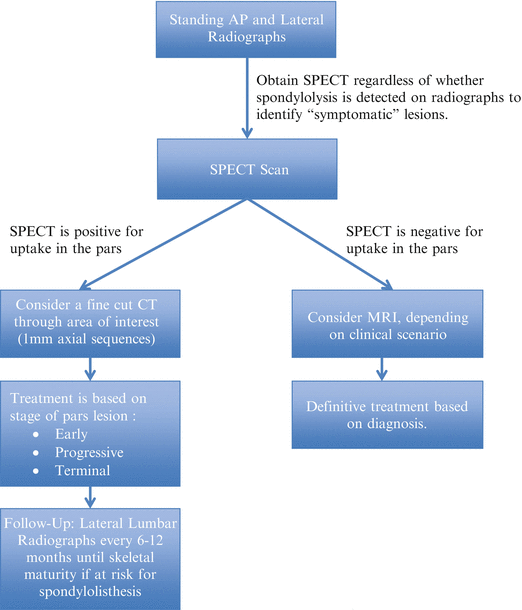

Fig. 6.5
Authors’ recommended diagnostic work-up for spondylolysis
Treatment
After diagnosing spondylolysis, the clinician must prepare to educate the patient and family regarding the etiology, pathophysiology, natural history, and available treatment options. A “one-sized-fits-all” approach risks alienating the athlete, who faces considerable internal and external pressures to return early to sport. Again, a thoughtful approach is necessary to support each athlete and his or her family in developing realistic treatment goals that maximize long-term functional outcomes (see Fig. 6.6 for a reasonable treatment algorithm). Any plan should account for the athlete’s age, noted biomechanical risk factors, training goals, sports participation, and severity of injury.
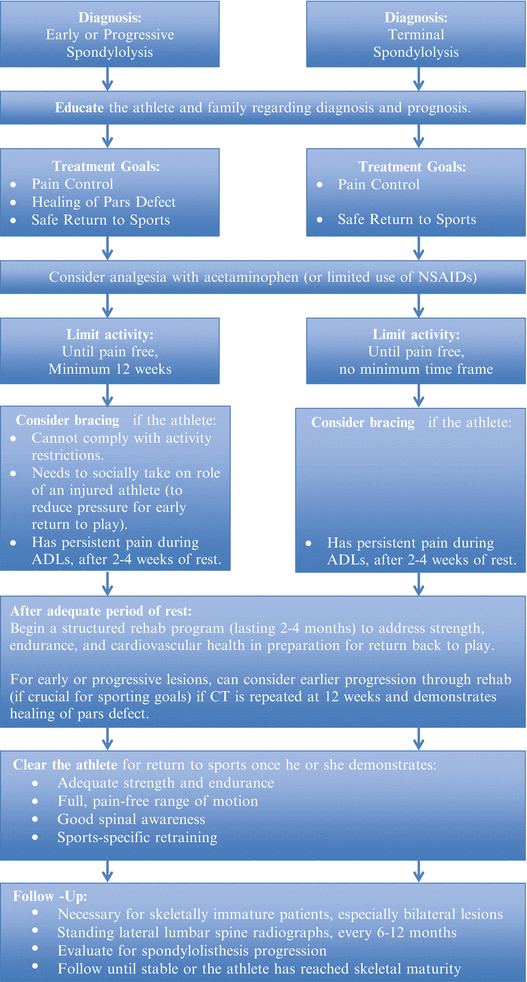

Fig. 6.6
Authors’ recommended treatment protocol for spondylolysis
Fortunately, most athletes do well with nonsurgical care (see section “Outcomes”). Despite variable treatment protocols cited throughout the literature, limiting physical activity beyond routine daily tasks is the mainstay of any treatment plan. However, some physicians also advocate for the universal use of a lumbosacral orthosis to facilitate bony healing. Unfortunately, there is little science to guide the prescription of one brace type over another (e.g., soft corset or rigid antilordotic orthosis) and limited consensus on optimal duration of use, which can range from a few weeks to an entire year [63].
Bracing
Bracing advocates emphasize its theoretical role in limiting mechanical stresses across the pars [69]. However, biomechanical studies have challenged this hypothesis, demonstrating that lumbosacral orthoses paradoxically facilitate, rather than restrict, lumbosacral segmental motion [70]. This is obviously problematic for treating spondylolysis, given most injuries occur at L5. The fact that lumbar orthoses are effective in limiting whole body motion [70, 71] may better account for the benefits seen in research and clinical practice [63].
Interestingly, studies have consistently demonstrated similarly favorable outcomes regarding pain control and bony healing regardless of the bracing type or protocol used [17, 30, 54, 72, 73]. One of the hallmark bracing studies performed by Steiner and Micheli retrospectively examined a cohort of 67 adolescents diagnosed with spondylolysis [54]. Each athlete was prescribed an antilordotic-modified Boston back brace to be worn 23 h per day. Sports participation was allowed in the brace if there was no pain. After an average 2.5-year follow-up, 78 % of the athletes had achieved good or excellent clinical results, while only 18 % had evidence of bony healing of the pars fracture.
Unfortunately, the study lacked a control group and did not stratify patients based on radiographic stage of injury, limiting the authors’ ability to comment on the concrete benefits of bracing compared with physical activity restriction alone [63]. This study and others demonstrate the disconnect between bony healing and clinical outcome [4, 17, 19, 30]. There has been no evidence to date through either comparative or cohort studies to demonstrate that bracing more safely or effectively facilitates earlier return to sports and, in certain patients, it may inadvertently reinforce a somatic focus on the injury. Coupled with the possible unnecessary financial costs involved with bracing, there is insufficient data to support uniform use in all athletes who develop spondylolysis.
Attempts to stratify which athletes experience the greatest rates of bony healing suggest that pars injuries are more likely to heal when an athlete presents with early stage, unilateral lesions found at levels L4 or higher [17, 66]. Fujii et al. demonstrated this well in their study of 239 pars defects treated with a soft corset, where they noted that only 3.5 % of the bilateral L5 “progressive” lesions achieved union compared to 100 % of the unilateral L4 “early” lesions [17]. But no study has definitively demonstrated a greater benefit of bracing over simple activity restriction, and studies investigating different bracing protocols have shown similar long-term outcomes even when athletes admit to not consistently using their brace [17, 54, 63, 72, 73]. Furthermore, a recent meta-analysis of 471 patients found that 89.0 % of subjects treated with a brace, compared with 85.8 % of subjects treated without a brace, achieved successful clinical outcomes (P = 0.75) [74].
Thus, many clinicians now emphasize the primary goal of helping an athlete to abstain from exertional activity when working to heal an early or, in some cases, a progressive symptomatic pars lesion or to decrease symptoms from a terminal pars defect. Bracing is then reserved for cases where an athlete experiences insurmountable pressure to maintain an active schedule, or if severe pain persists during activities of daily living despite several weeks of rest [63, 72], as there is no evidence that bracing improves bony healing. A recent study of 34 soccer players found this type of protocol to lead to good or excellent outcomes 94 % of the time [75]. Hopefully, future study in this area will help delineate whether there is any clear benefit of bracing over activity restriction alone and help clarify treatment algorithms based on stage or severity of injury.
Activity Modification and Rest from Sports
Although many questions remain in designing an optimal treatment protocol, there is little doubt that activity modification (e.g., not participating in sports), while difficult, is paramount for an athlete to achieve a favorable clinical outcome. In one hospital-based clinic, although athletes reported less than 50 % compliance with the treatment protocol, 86 % stayed away from sports for at least 3 months. Those athletes who rested from sports for at least 12 weeks were 16.4 times more likely to have the most favorable clinical outcome compared to athletes who returned early [72]. This is a simple and convincing statistic that can be used when counseling young patients and their families.
For athletes who have the potential to heal a pars defect, it is important to recognize that no studies have yet demonstrated healing of early or progressive lesions prior to 12 weeks [63, 76]. Thus, it is recommended that athletes with early or, in some cases, progressive lesions rest for at least 3 months. Symptomatic terminal lesions are unlikely to achieve bony union with nonsurgical treatment [17], and thus these athletes may begin return-to-play conditioning protocols once they achieve adequate pain control [63]. When earlier stage lesions are detected (e.g., when a CT is negative for pars lesions after a positive bone scan) a shorter period of rest could be considered, but there are no studies to guide precise management for this population [63].
Rehabilitation Stages
After an adequate period of rest, if the athlete is pain-free with full range of motion, a rehabilitation protocol ideally should be instituted prior to clearing the athlete to return back to sports. Generally, repeat imaging to verify healing provides little clinical benefit, except when an athlete has an urgent need to progress more quickly through a rehabilitative protocol [77]. Rehabilitation after spondylolysis should typically progress through three separate stages that correlate with specific goals in functional activity progression [78]. However, even during the pre-rehabilitation phase, the clinician should screen for psychosocial factors that may predispose to persistent pain or disability (e.g., anxiety, depression, catastrophizing behavior, passive coping styles) and work to implement relevant changes early in treatment.
The acute stage of rehabilitation should focus on low-impact cardiovascular activity and core strength training, with attention to biomechanical features that could predispose the athlete to future injury. Preserving range of motion, strength, and aerobic conditioning while maintaining spinal stability is the primary goal for the initial acute stage, which often lasts around 4 weeks [77, 79]. During this stage, athletes should focus on neutral spine stabilization techniques and learn to engage the deep stabilizing muscles along the spine, including the transversus abdominis and multifidi, without activating more global musculature or creating excess intersegmental motion [80]. Progressive loads are added through use of the limbs and eventually the athlete is taught to engage the core throughout the day with special attention to tasks that had previously caused pain. Initially, the athlete can be provided tactile, auditory, and visual feedback in a quiet environment with little consequence or distraction [79]. This can later translate to healthy movement patterns that become second nature or habit for the athlete during routine daily tasks.
The next stage, recovery, begins about 16 weeks after initiation of treatment for early or progressive lesions [77]. If there is a need to advance the athlete more quickly, this stage can be started as early as 12 weeks after initiation of treatment for an early pars fracture, as long as repeat CT imaging demonstrates a healed osseous union [77]. During the acute phase of rehabilitation, the athlete should develop comfort engaging and controlling his or her segmental spinal muscles, considered integral factors of core strength and stability. Thus, during recovery, physiotherapy shifts its focus to incorporate more advanced spinal stabilization techniques outside of the neutral plane. Work during this phase should include a thorough kinetic chain analysis while aerobic conditioning, strength training, and range of motion exercises are adapted and tailored to the athlete’s sport [77].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








