, James B. Galloway2 and David L. Scott2
(1)
Molecular and Cellular Biology of Inflammation, King’s College London, London, UK
(2)
Rheumatology, King’s College Hospital, London, UK
Abstract
Steroids, in the form of glucocorticoids, are often used in the management of inflammatory arthritis patients. They exert anti-inflammatory and immunosuppressive effects via a range of different mechanisms, with the end-effect being a reduction in disease activity. They are often given intramuscularly in the form of methylprednisolone (depomedrone) during the early stages of arthritis when disease activity is high or during flares of the disease. Long-term low-dose oral steroids, in the form of prednisolone, are used, although this is becoming less common in the biologic era. Although often effective at reducing arthritis activity and preventing erosive progression, their use is restricted by their broad range of side-effects. In this chapter we will discuss the different types and methods of steroid administration in inflammatory arthritis patients, the evidence supporting their use and their side-effects.
Keywords
CorticosteroidsPrednisoloneDepomederoneLocal InjectionSide-EffectsBackground
Steroids are given locally into joints or soft-tissues to treat synovitis, tenosynovitis and other local problems. They are given systemically as either high or low dose oral steroids by one or more intramuscular injections or by intravenous infusions. In each situation they have short-term efficacy. This benefit must be balanced against their long-term adverse events which can be serious. The clinical challenge is balancing their short-term benefits against their long-term risks [1].
The medical terminology is sometimes confusing. Using steroids to control inflammation involves exploiting their glucocorticoid properties. Many experts prefer to use the name glucocorticoids. Other steroids include mineralocorticoids, sex steroids and anabolic steroids. Nevertheless it is customary to use the generic term steroids when referring to glucocorticoids used to treat arthritis.
Pharmacological Effects
Steroids have complex anti-inflammatory and immunomodulatory effects. The term glucocorticoid reflects their involvement in glucose metabolism, which is a central effect of all glucocorticoids. Their anti-inflammatory and immunosuppressive effects involve a range of different mechanisms [2]. Steroids affect almost all immune cells. They inhibit white cell traffic and access to the sites of inflammation, interfere with the function of immune cells and suppress the production and actions of humoral factors. Many of their anti-inflammatory effects are mediated by cytosolic glucocorticoid receptors. These regulate proteins involved in inflammation and interfere with the function of transcription factors like nuclear factor-κB. Steroids also have rapid, non-genomic mechanisms, which are implicated in many of their immediate impacts.
In inflammatory arthritis steroids have impacts on the clinical symptoms of inflammation, which is partly due to infiltration of the synovium by lymphocytes. They also effect erosive progression, a process which is partly due to synovial infiltration by macrophages. Steroids influence the inflammatory process especially during the first months of treatment. They also have effects on erosions which are only evident after more prolonged treatment.
Steroids are metabolized in the liver. Consequently steroid effects are reduced by drugs that induce liver enzymes like phenytoin and rifampicin. Blood levels of steroids may rise in liver failure. Anticoagulant doses may need adjustment when given with steroids.
Cortisol, the classical glucocorticoid, is rarely used clinically. Synthetic glucocorticoids are preferred, which are more potent than cortisol. They differ in their pharmacokinetic properties, such as absorption and half-life, and the relative potency of their anti-inflammatory effects. Prednisolone is the dominant oral steroid and methylprednisolone (depomedrone) the dominant injectable steroid.
Effects on Joint Inflammation
Systemic steroids reduce joint inflammation in arthritis. They decrease the number of tender joints and swollen joints and reduce joint pain and elevated ESRs fall with treatment. A systematic review of the benefits of steroids report their benefits across 462 patients enrolled in 11 clinical trials [3]. The daily doses were 2.5–7.5 mg in four studies, 10 mg in three studies, and 15 mg in four. The low dose of steroids resulted in clinically significant improvements that lasted several months. Higher doses, such as 20 mg prednisolone daily, are also effective but are considered to have unacceptable risks of adverse effects.
Erosive Progression
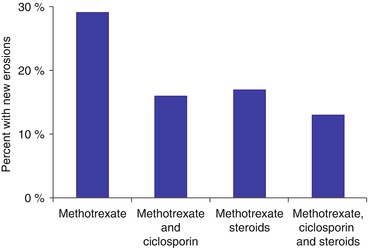
There is extensive evidence that steroids reduce erosive damage, particularly in early arthritis when given in combination with disease modifying anti-rheumatic drugs. Even low dose steroids achieve this goal. A systematic review of the impact of steroids on erosive progression in RA included 1,414 patients enrolled in 15 trials [4]. All the trials except one showed treatment benefits in favour of steroids. The proportion of benefit from steroids in reducing the progression of erosions from an average of all the studies over 1 year was 60 %. There is evidence for the steroids having a long-term benefit on erosive progression even if given for only 9 months or less. An example of the benefits of short-term steroids in early RA given with disease modifying drugs is shown in Fig. 10.1 [5].