CHAPTER 7 Spinothalamic system and viscerosomatic motor reflexes
functional organization of cardiac and somatic input
Introduction
Osteopathic manual manipulation is built upon the concept of reflex phenomena that involve the nervous system, viscera, and somatic tissues. Clinical studies and research reports have provided support for the existence of viscerosomatic and somatovisceral reflexes. These reflexes provide a two-way communication between the musculoskeletal system and the visceral organs as well as other systems. Moreover, manual manipulation is dependent not only on somatoautonomic processing and control via the nervous system, but also on the multiple and varied chemical routes of communication as well as the mechanical effects of musculoskeletal activity on viscera and on the movement of lymph and blood (Korr 1991). Although all these lines of communication are available, this chapter will address the mechanisms that are dependent on neural control related to the heart.
Normally cardiac function does not appear to be involved with the performance of the myotomes and dermatomes that are innervated by nerves from the upper thoracic spinal segments. In a similar manner, under normal circumstances the activities of the paraspinal musculature located on the upper thoracic segments do not appear to contribute to cardiac function. However, in pathological conditions such as ischemic heart disease, myocardial ischemia appears to link the heart with the upper thoracic somatic structures in a ‘self-sustaining circuit of autogenic impulses’ (Korr 1991). The result of this linkage is that pain is not felt as originating from the heart but is referred most commonly to the overlying somatic structures of the upper thoracic segments.
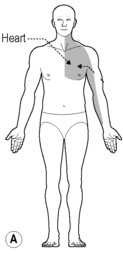
Patients with ischemic heart disease usually seek medical care when they experience the symptom of cardiac pain know as angina pectoris. The most typical manifestation is retrosternal pain described as crushing, burning, and/or squeezing (Bonica 1990, Harrison & Reeves 1968, Procacci & Zoppi 1989, Sampson & Cheitlin 1971) (Fig. 7.1A). Pain may also radiate to the throat, neck, or ulnar aspect of the left arm, sometimes reaching to the little finger. Less often, it radiates to the neck and jaw, or either the right or both arms. Angina pectoris may also be associated with the subjective sensation of anguish and fear of impending death. However, there is a great variability in the location of cardiac pain between patients and with the associated subjective sensations (Maseri et al. 1992). In addition to referred pain, functional changes can occur in muscle, skin, and bone.
Angina pectoris can also generate changes in the paraspinal muscles of the upper thoracic segments. Muscular changes resulting from the viscerosomatic reflex are characterized as a condition of exaggerated tone of the paraspinal muscles (Burns 1907). Furthermore, there appear to be at least two adjacent segments with confluent deep muscle splinting (Beal 1983). Theories in manual medicine have proposed that visceral spinal afferent pathways play a role in modifying the muscle tone of the upper thoracic paraspinal groups in response to changes in cardiac function (Beal 1989, Beal & Kleiber 1985, Cox et al. 1983, Larson 1976, Nicholas et al. 1985, 1991). Patients with cardiovascular disease have revealed a common spinal reflex ‘somatic dysfunction’ pattern involving spinal segments T1–T5, with the greatest incidence between T2 and T4 on the left side (Beal 1983, 1985, Beal & Kleiber 1985, Cox et al. 1983, Larson 1976) (Fig. 7.1B).
Spinal processing of cardiac nociceptive information
The section will address the neural mechanisms underlying angina pectoris by focusing on spinal processing of cardiac nociceptive impulses. Studies have shown that the C1–C2, C5–C6, and T2–T4 segments of the spinal cord are necessary for processing information in the neural hierarchy that regulates cardiac control (Foreman 1999). Spinothalamic tract cells and spinal neurons have been examined to study the effects evoked by electrical stimulation of the visceral spinal or vagal afferent fibers, the application of bradykinin or other algesic chemicals into the heart or into the pericardial sac, occlusion of the coronary arteries, and stimulation of the skin and muscle in various animal models.
Activation of cardiac nociceptors
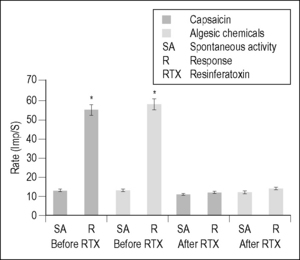
Angina pectoris often results from ischemic episodes that excite chemosensitive and mechanoreceptive receptors in the heart (see Coleridge & Coleridge 1980, Foreman 1999, Longhurst et al. 2001 for review). Ischemic episodes release a barrage of chemicals, including adenosine and bradykinin, which excite receptors of the visceral spinal afferent and vagal afferent pathways. The discovery of the chemicals that are released during periods of myocardial ischemia and activating spinal afferent fibers innervating the heart may be important for treating angina pectoris; however, there is very little information about the receptors involved. Recently, it has been proposed that the onset of angina pectoris associated with myocardial ischemia may depend on the activation of the transient receptor potential vanilloid-1 (TRPV1). This receptor is a molecular integrator of noxious stimuli that is specifically expressed in the plasma membrane of nociceptive afferent fibers and opens an important nonspecific cation channel that is activated by capsaicin (CAP), heat stimuli, and protons (Caterina et al. 1997, Pan & Chen 2004). It has been shown that TRPV1-expressing afferent nerves are distributed extensively on the epicardial surface of the rat ventricle (Zahner et al. 2003). Epicardial application of CAP excites visceral spinal afferent fibers and produces sympathoexcitatory reflexes (Pan & Chen 2004, Schultz & Ustinova 1998, Zahner et al. 2003); TRPV1 antagonists can eliminate these effects. Injections of CAP into the left atrium or pericardial sac also activate spinal and spinoreticular neurons in cats (Bolser et al. 1989) and rats (Qin et al. 2006). These results suggest that excitation of TRPV1-containing visceral spinal afferent fibers might excite spinal neurons that receive convergent input from the heart and overlying somatic structures. To support this suggestion, we have shown that the majority of upper thoracic spinal neurons with cardiac input responded to intrapericardial administration of BK or CAP and received somatic input from structures overlying the heart (Qin et al. 2006). To determine whether TRPV1-containing fibers were responsible for transmitting this cardiac nociceptive information, resinferatoxin (RTX), an ultrapotent analog of CAP, was used to desensitize the fibers (Karai et al. 2004, Pan et al. 2003, Raisingani et al. 2005, Szallasi and Blumberg 1989, Wu et al. 2006, 2007). RTX causes TRPV1 to become permeable to cations, specifically Ca2+ ions, and evokes a powerful sensitization that is followed within 20 minutes by desensitization and analgesia.
Desensitization of cardiac visceral spinal afferent fibers containing TRPV1 with intrapericardial RTX eliminated excitatory neuronal responses to BK, CAP, and also to a cocktail of algesic chemicals (BK, serotonin, PGE2, histamine, and adenosine) released during myocardial ischemia (Foreman 1999, Meller & Gebhart 1992, Qin et al. 2006) (Fig. 7.2). However, selective blockade of TRPV1-containing afferent fibers with intrapericardial capsazepine significantly attenuated the activation of spinal neurons by CAP but did not affect neuronal responses to BK or to somatic manipulation. These results provided evidence that CAP-sensitive visceral spinal afferent fibers play an important role in the activation of upper thoracic spinal neurons by cardiac noxious stimuli. However, the BK-elicited spinal neuronal responses are not dependent upon TRPV1 receptors at the nerve endings of cardiac afferents. It has been proposed that visceral spinal afferent fibers containing TRPV1 may operate as molecular sensors, because this receptor and receptor channel complex can activate cardiac nociceptors by detecting the release of BK, ATP, serotonin, and lipid metabolites as well as changes in pH that commonly occur with tissue ischemia (Julius & Basbaum 2001, Pan & Chen 2004). In summary, these new findings are important to advance our understanding about the afferent mechanisms that may contribute to angina pectoris and the phenotypes of visceral spinal afferent neurons that are involved in the activation of sympathetic reflexes to myocardial ischemia.
Neural mechanisms of referred pain in upper thoracic spinal cord
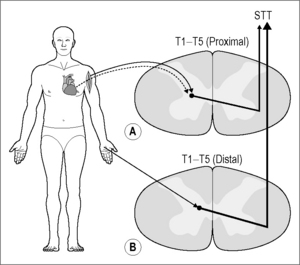
Visceral spinal afferent fibers from the heart enter the upper thoracic spinal cord and synapse on cells of origin of ascending pathways. This review focuses on the spinothalamic tract (STT), but other pathways are excited as well (Foreman 1999). The responses of individual STT cells, which have their origin in the gray matter of the thoracic spinal cord, to nociceptive input from the heart have been assessed by transient coronary artery occlusion or injection of algesic chemicals into the heart, followed by examination of somatic fields (Fig. 7.3) (see Foreman 1999). The STT projects to the medial and lateral thalamus and, based on positron emission tomography studies, activates several cortical areas, including the anterior cingulate gyrus, the insula, the lateral basal frontal cortex, and the mesiofrontal cortex (see Foreman 1999, Jänig 2006, Chapter 2).
In general, three main characteristics can be used to describe angina pectoris:
Convergence of somatic and visceral input
Chemical or electrical activation of cardiac visceral spinal afferent fibers excites approximately 80% of the STT cells located in the lamina I and laminae V–VII of the T1–T6 spinal segments (Ammons et al. 1985, Blair et al. 1982, Hobbs et al. 1992) (Fig. 7.4A, B).
It should be noted that these afferent fibers are most likely polymodal, and when excited they activate protective reflexes, regulate cardiac function, and elicit pain (see Jänig 2006). These STT cells also receive somatic input from the chest and upper arm (Fig. 7.4C). In addition, approximately 60% of the STT neurons in the C5–C6 segments also receive afferent input from the heart (Hobbs et al. 1992). It is important to note that STT cells in the cervical enlargement (C7, C8) are activated by somatic input from the distal forelimb and hand but receive very little input from activation of the cardiopulmonary fibers (Hobbs et al. 1992). These results might explain the clinical observations that angina pectoris generally is not referred to the distal forearm and hand (Harrison & Reeves 1968, Procacci & Zoppi 1989, Sampson & Cheitlin 1971).
Anatomical studies have shown that cardiac visceral spinal afferent fibers generally enter the T1–T5 segments but not the C5–C6 segments (Hopkins & Armour 1989, Kuo et al. 1984, Vance & Bowker 1983). Thus, information transmitted in the upper thoracic spinal visceral afferent fibers may activate a propriospinal pathway that makes synaptic connections with the C5–C6 STT cells (Nowicki & Szulczyk 1980) and/or branches of the T2 and T3 afferent fibers may travel for several segments in the zone of Lissauer and then terminate on C5–C6 STT neurons (Sugiura et al. 1989). This last possibility is less likely because the branches would need to bypass the cervical enlargement in order to reach the upper cervical segments. However, it is possible that during fetal development neurochemical messages from cells in the cervical enlargement (C7–C8) may prevent branches from forming synapses in these segments, but at present this is speculative. In summary, visceral and somatic input converges onto a common pool of STT cells; this provides a substrate to explain pain referred to overlying somatic structures.
Referral to proximal and axial somatic structures
Human studies have shown that the pain of angina pectoris generally radiates to the chest more than 95% of the time and to the left proximal shoulder 30–60% of the time. Pain is felt much less frequently further down the arm (Bennet & Atkinson 1966, Sampson & Cheitlin 1971, Sylvén 1989). It has been shown that stimulation of the cardiopulmonary visceral spinal afferent fibers strongly activated approximately 80% of the C5–C6 and T1–T5 STT cells receiving input from proximal somatic receptive fields, but only 35% of cells with distal somatic input were weakly excited (Hobbs et al. 1992). These results support the clinical observations that angina pectoris most commonly radiates to proximal axial somatic structures.
Muscle-like pain
Generally, angina pectoris is described as a deep, diffuse, dull, and aching type of pain. Muscle pain often is described in the same way. In contrast, cutaneous pain is usually described as sharp and well focused. Similarities between visceral pain and muscle pain have been shown in experiments conducted in patients suffering from angina pectoris (Kellgren 1937-38, 1940). Patients were asked to compare their angina pectoris pain with pain generated by injecting a hypertonic saline solution into the muscles surrounding the interspinous ligament of the left eighth cervical or first thoracic segment. These patients noted that the onset, continuation, character, and segmental localization of the muscle pain were similar to the pain they experienced with angina pectoris (Kellgren 1937–38). Clinical and experimental studies have shown that diseases of visceral organs result in hyperalgesia of the overlying muscle. In patients calculosis of the upper urinary tract leads to the development of muscle hyperalgesia, but with less cutaneous involvement (Giamberardino et al. 1994, Vecchiet et al. 1989). Experimental studies have also shown that noxious stimulation of the ureter results in central sensitization of spinal neurons and muscle hyperalgesia (Giamberardino et al. 1996, 1997, Laird et al. 1996). The development of muscle hyperalgesia described in these studies may depend on viscerosomatic motor reflexes in a manner similar to the changes in the tonicity of thoracic paraspinal muscles resulting from activation of cardiosomatic reflexes, as described in the introduction and in a subsequent section.
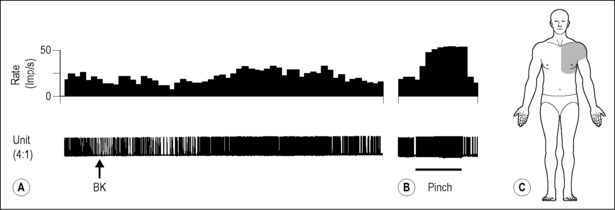
Studies conducted to examine segments processing noxious cardiac input in the STT cells of the upper thoracic spinal cord supported the findings that pinching the proximal muscles can generate a strong noxious input (Hobbs et al. 1992). The predominant somatic afferent input to STT cells that are excited by noxious cardiac visceral spinal afferent input originates from pinching the proximal muscle (Fig. 7.5A). In contrast, noxious cutaneous pinch alone in the chest and proximal somatic receptive fields of the upper arm generated a much smaller response. However, cutaneous afferent input resulting from pinching the distal receptive fields of the fingers and hands produces the greatest responses in another population of STT cells that received minimal if any convergent cardiopulmonary visceral spinal afferent input (Fig. 7.5B). In summary, visceral spinal afferent input from cardiopulmonary fibers converged onto STT cells with proximal muscle input, whereas visceral afferent input has minimal effect on STT cells receiving primarily distal cutaneous inputs (Fig. 7.5). As a result of this arrangement, nociceptive input generated during myocardial ischemia and infarction most likely mimics muscle pain. Thus, angina pectoris is most commonly felt as a deep, diffuse, aching pain that is generally referred to proximal and axial structures such as muscle, ligaments, and tendons.
Neural mechanisms of referred pain in the upper cervical spinal cord
Osteopathic literature, dental case studies, and interruption of cardiac spinal visceral afferent fibers to treat angina pectoris led us to examine an explanation for pain referral to the neck and jaw region during myocardial ischemic episodes. Manual medicine physicians have shown in a population of patients with cardiovascular disease that the segmental distribution of increased paraspinal tone in the majority of them was from segments T1 to T5, however, a few of the patients had paraspinal tonicity changes in the C1–C3 segments (Beal 1985, Fig. 7.1B). Articles in the dental literature also report that myocardial ischemia elicits craniofacial pain as the only complaint in approximately 6% of dental patients (Kreiner & Okeson 1999, Kreiner et al. 2007, Tzukert et al. 1981). A recent case report has suggested that the vagal afferents may mediate angina pectoris expressed as jaw and tooth pain (Myers 2008). Early clinical observations of neck pain being unmasked after sympathectomy (to interrupt spinal visceral afferents from the heart) to reduce angina pectoris led to the hypothesis that STT cells in the C1–C2 region receive cardiac input (Lindgren & Olivecrona 1947, White et al. 1933, White & Bland 1948). Thus, these observations served as a basis for exploring neural mechanisms of referred pain in the cervical spinal cord. To address this hypothesis recordings of extracellular potentials were made from STT cells located in the C1–C2 spinal segments (Chandler et al. 1999, 2000). Coronary occlusion, injection of algesic chemicals into the heart before and after bilateral vagotomy, or electrical stimulation of cardiopulmonary visceral spinal afferent fibers and thoracic vagal afferents were used to activate the neurons.
Electrical stimulation of vagal and cardiac sympathetic nerves showed that STT and non-STT spinal neurons in C1–C2 were more responsive to stimulation of vagal afferents than of spinal cardiac afferents, and that the somatic fields for these cells were located primarily in the jaw and neck regions (Chandler et al. 1996, Qin et al. 2001). In addition, vagotomy markedly reduced the nociceptive input produced by algesic chemicals injected into the heart, as evidenced by reduced activity of STT and non-STT spinal neurons in the cervical region (Chandler et al. 2000, Qin et al. 2001) (Fig. 7.6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree