CHAPTER 24
Spasticity Due to Multiple Sclerosis: Epidemiology, Pathophysiology, and Treatment
Anjali Shah and Ian Maitin
SPASTICITY IN MULTIPLE SCLEROSIS
Multiple sclerosis (MS) is a chronic, debilitating, inflammatory disease of the central nervous system. There is no cure for the disease, and management of it includes the use of disease-modifying therapies and symptomatic agents to reduce and/or prevent relapses and disease progression. MS affects approximately 400,000 persons in the United States (1), with an estimated prevalence of 1 in 1,000 individuals in North America and is one of the most common causes of disability in young adults. The symptoms of MS are numerous and include weakness, paresthesias, visual changes, fatigue, cognitive dysfunction, ataxia, and spasticity. Patients with MS report that their spasticity has a significant detrimental effect on their lives. A survey of 1,554 self-reporting people with MS residing in the United Kingdom demonstrated that 82% experience spasticity and 54% classified the impact of spasticity as “high” or “moderate” (2). Greater than 80% of patients with MS report some degree of spasticity, with one third of these modifying or eliminating daily activities as a result (3).
Spasticity is a disorder of increased resistance of a muscle, or group of muscles, to an externally imposed stretch, with more resistance to rapid stretch (4,5). What differentiates spasticity from other components of the upper motor neuron syndrome (UMNS) is its relationship with the velocity of movement. A rigid muscle displays the same mechanical properties whether it is stretched slowly or quickly. Spasticity in the patient with MS ultimately leads to a detrimental increase in disability resulting in increased energy requirement for daily activities and decreased quality of life (6,7).
Due to the distribution of plaque in the brain and spinal cord, patients with MS-related spasticity frequently present clinically similar to patients with stroke, traumatic brain injury (TBI), or spinal cord injury (SCI). Treating spasticity in the person with MS poses unique challenges due to the progressive nature of the disease. Symptoms may fluctuate day to day, and are also dependent on the temperature, time of day and fatigue level. Thus, unlike those patients who have static injuries, MS-related symptoms vacillate frequently by nature of the disease process. This logic extends to include spasticity. The clinician and the patient must be aware of this to allow for varying dosages of medications or treatments at different points in the disease.
It is important for the clinician to be aware of the profound effect fatigue and cognitive dysfunction have on people with MS. Patients will frequently forego spasticity treatment options if their fatigue or cognitive function is compromised. It is crucial for the clinician to treat spasticity adequately without worsening other symptoms.
The goal of this chapter is to review the etiology, pathophysiology, diagnosis, and evaluation of spasticity in this patient population. In addition, treatment and management strategies of MS-associated spasticity are reviewed with emphasis on those items that are unique to this clinical population.
EPIDEMIOLOGY
About 1 in 1,000 persons is affected with MS in North America (1), with a worldwide prevalence of more than 1,000,000 persons. Females are affected about twice as frequently as males; diagnosis typically occurs between ages 20 and 40 years, and first-degree relatives of a person with MS have approximately 2% to 5% risk of being diagnosed with MS (8). The advent of magnetic resonance imaging (MRI) has allowed for earlier diagnosis. MS was originally deemed a disease of Northern European descent and more prevalent in populations farther from the Equator; however, MS now affects persons of all races and ethnicity and the latitude gradient of MS is believed to be decreasing (9,10). Whites remain the most commonly affected group.
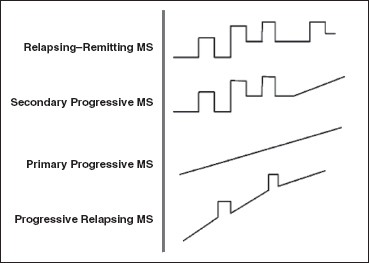
FIGURE 24.1 Four main subtypes of MS. MS, multiple sclerosis.
There are four main types of MS (Figure 24.1):
• Relapsing–remitting—This is the most common form of MS and affects approximately 85% of patients. Individuals have relapses or exacerbations with remissions during which time some or all of their disability recovers to their baseline. Females are affected twice as frequently as males in this form.
• Secondary progressive—These individuals experience relapsing–remitting MS initially and then begin to have increasing amounts of disability accumulation or progression with no remission. All relapsing–remitting patients will convert to this form at some stage of their disease.
• Primary progressive—This subtype is characterized by increasing disability accumulation over time without periods of relapses or remissions. The course and severity vary among individuals. Currently, there are no disease-modifying agents for this type of MS. Males and females are equally affected, and about 10% of MS patients have primary progressive MS.
• Progressive relapsing—This is the most aggressive and rarest type of MS. Approximately 1% to 5% of patients are affected. The disease course is characterized by rapid accumulation of disability with or without periods of remission.
The North American Research Committee on Multiple Sclerosis conducted a survey studying the prevalence of spasticity as well as its relationship to functional activity. The survey results reported that 84% of patients reported some degree of spasticity ranging from mild to completely incapacitating (3). In addition, patients with higher levels of spasticity were more likely to be male, disabled, and unemployed compared with those with reduced amounts of spasticity. The study found a linear correlation in the amount of spasticity and degree of disability ranging from ambulatory to bedbound.
PATHOPHYSIOLOGY
Normal muscle does not display resistance to passive movement, nor does it exhibit electromyographic activity. A spastic muscle will have abnormal muscle activity with slow or fast stretch movements (11). Spasticity is a component of the UMNS, which also includes increased spinal reflexes, muscle hyperactivity, flexor spasms, and disordered control. Although the pathophysiology remains a subject of debate, spasticity appears to occur as a result of spinal, supraspinal, and/or cerebral dysfunction. The result is an imbalance between classic inhibitory (dorsal reticulospinal) and excitatory (mostly bulbopontine tegmentum) pathways that leads to abnormal proprioceptive input in the spinal cord (11–14). The clinical presentation is largely dependent on the anatomic site of injury or insult.
In MS, spasticity can be due to lesions in the brain, spinal cord, or both (4), with lower limb spasticity almost twice as prevalent as upper limb spasticity (97% vs 50%) (6). One of the most common questions asked by a person who is newly diagnosed with MS is if the ability to ambulate will be affected and how quickly he or she will be in a wheelchair. Adequate assessment and timely treatment of spasticity can help ensure long-term ambulatory and functional ability of the patient with MS.
In addition to receiving input from the muscle spindle, motor units receive information and are influenced by proprioceptive, enteroceptive, and suprasegmental pathways. Noxious stimuli, such as pain, distended bladder, renal calculi, constipation, ingrown toenail, infection, or pressure ulcers, as well as nonnoxious stimuli (yawning, transferring), can trigger exacerbation of spasticity or other components of the UMNS. Patients who complain of sudden worsening should be screened for the presence of aforementioned noxious stimuli before making more aggressive changes to their treatment.
NEGATIVE EFFECTS OF SPASTICITY AND REASONS TO TREAT
Spasticity can have deleterious effects on the performance of numerous tasks that are important to one’s daily living. When compared with healthy subjects, patients with gait abnormalities secondary to MS-related spasticity demonstrated increased cost of walking in the 10-m walking test (6). The increased cost of walking, coupled with a population with decreased cardiorespiratory systems, frequently results in diminished endurance for ambulation or other activities.
The involvement of spasticity in one or more limbs can lead to immobility and/or inactivity. The long-term effects of immobility have been well characterized in several studies involving prolonged bedrest (15,16). Prolonged periods of bedrest have been reported to be detrimental and could potentially lead to further complications to multiple body systems (17). Immobility can lead to disuse atrophy and loss of strength and endurance in the musculoskeletal system. This is commonly seen in the patient with muscle atrophy in the involved spastic limb(s). People who are on bedrest may lose 10% to 15% of their muscle mass in 1 week and the time required to regain this bulk is frequently two to three times the duration of their bedrest (18). Daily isometric exercises focusing on the gastrocnemius, soleus, and tibialis anterior muscles should be encouraged to maintain muscle mass. Lower limb exercises should be performed regularly because these muscles lose significantly more muscle mass with inactivity compared with the upper limbs.
Collagen comprises 20% of total body mass and is the most abundant protein in the body (19). During periods of inactivity or immobility, the connective tissue properties of tendons and ligaments change drastically and affect the collagen and proteoglycan properties of soft tissue. This can lead to decreased gliding and lubrication of tendons and ligaments resulting in myogenic and soft-tissue contractures. This can profoundly compromise one’s independence and may necessitate assistance for transfers, toileting, bathing, and dressing. Patients should be encouraged to stretch daily for 15 minutes with focus on the spastic limbs (20). Patients who are bedbound would benefit from active and passive range of motion (ROM) exercises. They may require hospital beds that are equipped with side rails and an overhead trapeze so that they can conduct some position changes independently.
Bone mineral status is affected by immobility (21,22). Repeated loading will increase bone mass, whereas decreased loading will negatively affect the mass. In addition, patients with MS are frequently treated with intravenous or oral steroids for exacerbations or maintenance therapy, which adds additional risk for bone mineral decline. Glucocorticoids are the most common form of drug-related osteoporosis. They should be counseled to perform weight-bearing exercises (standing daily for 15–20 minutes either alone or with assistance of a walker, tilt table, or standing frame). For those who are unable to stand, isometric exercise and supplementation with calcium, vitamin D, and bisphosphonate therapy is strongly recommended.
Immobility due to spasticity will also compromise cardiovascular and pulmonary status. Periods of prolonged immobility can increase heart rate by 1 beat/min every 2 days (23). Patients may acquire immobilization tachycardia and reduced exercise tolerance. Stroke volume and maximal oxygen uptake by the heart and skeletal muscles decrease in as little as 2 to 3 weeks of inactivity (24).
Adequate and timely treatment of spasticity can prevent compromise of the cardiovascular, pulmonary, and musculoskeletal systems. In addition, preservation of bone mineral density status is maintained. Finally, through the aforementioned preventive measures, prevention of long-term complications of inactivity such as pressure ulcers, contractures, and deep venous thrombosis formation secondary to spasticity can occur.
EVALUATING SPASTICITY
Although spasticity is generally recognized as a symptom of MS, it is frequently not adequately controlled or assessed in routine office visits (4). Possible factors for this include lack of awareness of the potential negative impact of spasticity by the clinician and the patient, limited office visit time, and ignorance about the multiple treatment options available. A study of people with MS in England reported that about 50% of patients were either on a suboptimal dosage of their oral antispasmodic medication or not treated at all (6). Only 32% of the group reportedly had adequate treatment of their spasticity. Clinicians are encouraged to question and assess the presence of increased muscle tone of patients with MS at their visits.
The Consortium of Multiple Sclerosis Centers recommends that spasticity be screened as part of routine visits for patients with MS. In addition to evaluating a patient’s strength, ROM, deep tendon reflexes, and muscle tone with the patient seated on the examination table, the patient should be examined in a dynamic setting such as observing the gait pattern and how the hand intrinsics flex and extend while picking up an object and looking for evidence of cocontraction. The patient may also report difficulty falling or staying asleep due to increased muscle spasticity at night.
In addition to the clinical examination, spasticity can be assessed based on clinical scales, neurophysiologic testing, or biomechanical techniques (25). No single measurement has demonstrated superiority over the other, as the evaluation and assessment of spasticity can focus on several areas including ROM, resistance, and speed, which can be subjective or objective evaluations. The varying number of scales available for spasticity make study comparison challenging, as it is not unusual to see different scales used in papers dealing with the evaluation and management of spasticity.
Multiple Sclerosis Spasticity Scale
The Multiple Sclerosis Spasticity Scale is an 88-item self-reported questionnaire (25). It was generated through a series of two postal surveys, face-to-face interviews with people with MS, and focus groups of MS specialists, patients, and other health professionals involved in the care of this treatment population. The authors validated the scales in two large groups of persons with MS. The questionnaire is designed to provide information about the impact of spasticity on a patient’s mobility, pain, activities of daily living (ADL), emotions, and social functioning. It consists of eight subscales that can be used alone or in conjunction with each other. The Multiple Sclerosis Spasticity Scale is unique in providing information about the multiple ways spasticity can affect a patient. It can be used to complement clinical scales (summarized subsequently) that clinicians frequently use to score the level of spasticity involvement.
Ashworth and Modified Ashworth Scales
Spasticity assessment is most commonly performed using the Ashworth Scale (AS; Table 24.1) and the Modified Ashworth Scale (MAS) (Table 24.2) (26). The AS was developed in 1964 (27). The MAS was developed in 1987 in response to concerns by the authors that the “Ashworth grade of ‘1’ was indiscrete” (28). Both scales are ordinal scales with scores ranging from 0 to 4. The MAS has an additional value of 1+ and has demonstrated interrater reliability of 86.7% in assessment of elbow flexor muscle spasticity. Both scales have been criticized for their lack of sensitivity to subtle changes in spasticity and therefore exhibit challenges in demonstrating clinical effect in trials.
TABLE 24.1
ASHWORTH SCALE | |
0 | Normal tone |
1 | Slight hypertonus, a “catch” when limb is moved |
2 | Mild hypertonus, limb moves easily |
3 | Moderate hypertonus, passive limb movement difficult |
4 | Severe hypertonus, limb rigid |
Source: Ref. (27). Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542.
TABLE 24.2
MODIFIED ASHWORTH SCALE | |
0 | No increase in tone |
1 | Slight increase in tone, manifested by a catch and release or by minimal resistance at the end of the ROM when the affected part(s) is moved in flexion or extension |
1+ | Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM |
2 | More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved |
3 | Considerable increase in muscle tone, passive movement difficult |
4 | Affected part(s) rigid in flexion or extension |
ROM, range of motion.
Source: Ref. (28). Bohannon RW, Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207.
The Numeric Rating Scale (NRS) is a patient-rated 0 to 10 assessment that has been found to be reliable and valid for measuring spasticity. It is thought to be more sensitive to changes that are meaningful to the patient, in contrast to clinician-rated measures of spasticity that tend to be more objective but less focused on the patient’s experience. The NRS has demonstrated moderate to high levels of correlation between patient reported spasticity and clinician administered assessment methods (29).
Tardieu Scale
The Tardieu Scale evaluates muscle response to velocity and movement quality (30). It adds the response to muscle moved at three different speeds. The original scale was developed in 1954 and has undergone several revisions. The following three factors are involved in the Tardieu Scale when assessing spasticity: (a) strength and duration of the stretch reflex, (b) angle at which the stretch reflex is activated, and (c) the speed necessary to trigger the stretch reflex (31). It is also an ordinal scale that is graded from 0 to 4. The patient should be seated for upper limb evaluation, whereas supine position is recommended for lower limb testing (32).
Penn Spasm Frequency Scale and Spasm Frequency Scale
The Penn Spasm Frequency Scale is a self-reported scale that was developed to assess spasticity in patients with MS and SCI after insertion of an intrathecal baclofen (ITB) pump (Table 24.3) (33). The scale ranks the occurrence of spasms by scoring as follows: 0, no spasm; 1, mild spasm induced by stimulation; 2, infrequent full spasms occurring less than 1 per hour; 3, spasms occurring more than once per hour; and 4, spasms occurring more than 10 times per hour (14). A variation to the Penn Spasm Frequency Scale is the spasm frequency scale that ranks the number of spasms occurring by day instead of by the hour (34).
Timed Up-and-Go Test
The Timed Up-and-Go Test is a functional assessment that requires a person to stand from a chair, walk 3 m, turn around, walk back to the chair, and return to a seated position (35). The patient performs the test at a comfortable pace and may use any required assistive device. Timing of the test commences as the patient stands and is complete with a return to the chair. The normal duration of the timed up-and-go test is 7 to 10 seconds. Patients requiring greater than 20 seconds are considered to have functional mobility problems. The test is validated, reliable, and correlated with the Berg Balance Scale.
Ambulation Index
The Ambulation Index (AI) was developed for patients with MS. It assesses a patient’s ability to walk a 25-ft distance rapidly and safely (36). The time required to complete the AI and assistive device used are documented. Scores range from 0, which indicates an independent ambulator, to 10, which indicates a bedridden patient. Similar to the AS and MAS, the AI is not always sensitive to changes in focal spasticity.
TABLE 24.3
PENN SPASM FREQUENCY SCALE | |
Score |
|
0 | No spasm |
1 | Mild spasm at stimulation |
2 | Irregular strong spasms occurring <1 per hour |
3 | Spasms occurring >1 per hour |
4 | Spasms occurring >10 per hour |
Source: Ref. (33). Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–1521.
Berg Balance Scale
The Berg Balance Scale is a 14-item scale that assesses balance by means of a series of tasks, which require a patient to hold postures for a given duration (37). It is a 5-point ordinal scale, with 0 the lowest and 4 the highest score. Points are deducted for patients who cannot hold a position for the required time, as well for patients who lose their balance and require assistance. The test takes approximately 10 minutes to administer. Scores are stratified according to fall risk as follows: 41 to 56, low fall risk; 21 to 40, medium fall risk; 0 to 20, high fall risk. A score of 45/56 is considered the cutoff for safe independent ambulation.
Expanded Disability Status Scale
The Expanded Disability Status Scale (EDSS) is an ordinal scale ranging from 0 to 10 that is utilized in assessment of disability in people with MS (38). A score of 0 indicates no disability, and a score of 10 is death due to MS. There are eight functional systems (FS) included in the EDSS: visual, pyramidal, cerebellar, brainstem, sensory, bowel and bladder function, cognition, mental, and other. In addition to assessing the eight FS, a patient’s ambulation ability and reliance on an assistive device are assessed. A patient’s score on each FS is evaluated in conjunction with his or her ambulatory ability for the score. In general, scores of 0 to 4.0 mean patients can ambulate with no assistance and the FS score has greater influence over the overall scale. An EDSS score of 4.0 to 7.5 indicates dependence on an assistive device with limited ambulation distance. A score of 6.0 or greater indicates reliance on at least a single point cane. Scores of 7.5 to 10.0 are determined by the patient’s ability to transfer from a wheelchair to bed.
TREATMENT
Prevention
People with MS and their caregivers should receive an education about the risk of developing spasticity and suggested methods to prevent the onset of spasticity. Suggested factors that could negatively affect spasticity are listed in Table 24.4. It is recommended that patients stretch frequently, even before the onset of spasticity, to prevent or delay the onset of changes in muscle tone or weakness. For patients with limited mobility, caregivers are encouraged to perform passive stretches of commonly affected muscles on a daily basis because this patient population is at greater risk of contracture and decubitus ulcer formation. There are several online resources available that demonstrate stretches and exercises for patients with MS. Interestingly, there is no research study of the effects of exercise on the ramifications of spasticity in this population.
TABLE 24.4
POTENTIAL SPASTICITY PRECIPITATING FACTORS IN PATIENTS WITH MS |
Noxious stimuli |
MS, multiple sclerosis.
Source: Ref. (4). Haselkorn JK, Balsdon Richer C, Fry Welch D, et al. Overview of spasticity management in multiple sclerosis. Evidence-based management strategies for spasticity treatment in multiple sclerosis. J Spinal Cord Med. 2005;28:167–199.
Rehabilitative Strategies
There are several rehabilitative interventions available for spasticity management. The Consortium of Multiple Sclerosis Centers panel on spasticity recommends the use of skilled rehabilitation strategies for its management. A summary of possible therapeutic interventions is listed in the following discussion.
Range of motion and stretching. Muscles that cross two joints (eg, lumbricals, gastrocnemius, iliopsoas, and hamstring) are most at risk of the development of spasticity. Daily stretching to maintain full ROM of these muscles is suggested. The lower limbs are more frequently involved and stretching of the heel cord and hamstrings with Thera-bands is encouraged (6). Stretches should be sustained for at least 30 seconds and done multiple times in each leg.
Strengthening. The development of spasticity can lead to decreased activity and muscle weakness. Although no specific recommendations exist for people with MS, therapies should be prescribed based on a person’s abilities and can include isometric exercises, progressive resistance exercises, endurance exercises, and core body strengthening. No specific type of exercise has been proven superior in this population.
Stroking. One small pilot study that included 10 people with MS-related plantar flexor spasticity evaluated the effects of “slow stroking” over the lower limbs for the management of their spasticity (39). H-reflex activity was measured at prestroking, immediate poststroking, and 30 minutes poststroking. There was a 30% decrease in H-reflex amplitude from baseline, reflecting reduced spasticity. Light pressure may facilitate decreases in spasticity; however, larger studies are necessary.
Heat therapy. Approximately 80% of patients with MS exhibit heat insensitivity, which can lead to the temporary development of new symptoms or worsening of current symptoms (40). In general, for people with MS, function generally deteriorates with heat and improves with cold (41). As a result, heating modalities are generally not recommended as a treatment in this population (4).
Cold therapy. Muscle cooling has been found to reduce muscle stretch activity and clonus (42). Cooling demyelinated nerves can reduce conduction block and transiently improve nerve conduction (43), as well as reduce fatigue in patients with MS. Fifteen minutes of muscle cooling before engaging in heavy physical activity is recommended to reduce spasmodic activity (44); however, it should be noted that the immediate effect of cooling is a transient increase in muscle tone. The use of cooling suits can reduce core body temperature by 0.2°F to 1.8°F (45). In addition to the traditional cooling vest, other cooling devices such as hats, bandanas, seats, and pillows are also commercially available. Aquatic therapy in swimming pools that are approximately 80°F to 82°F is recommended to stretch spastic muscles and for cardiovascular and muscle-strengthening therapeutic interventions (4). Swimming pools maintained at a temperature greater than 85°F should be avoided at all times. It is critical that therapists should be cautioned to ensure that the patient’s sensory system is intact before engaging in heating or cooling applications.
Transcutaneous electrical nerve stimulation. Transcutaneous electrical nerve stimulation (TENS) is used to control pain in several disorders, and the utility of TENS units demonstrated spasticity reduction in spasticity secondary to stroke and SCI (46,47). Armutlu et al (48) reported significant reduction in plantar flexor spasticity in 10 patients with MS using high-frequency (100 Hz) TENS unit for 20 minutes daily for 4 weeks. Reduction was noted on MAS, electrophysiologic measurements, and the AI (36). Long-term effects on spasticity after discontinuation of TENS application was not commented on in this study.
Another study assessed the utility of TENS for management of spasticity using a crossover design. Thirty-two subjects were divided and received either 60 minutes or 8 hours of high-frequency (100 Hz) TENS treatment daily for 2 weeks (49). No reduction in spasticity was found in either group. Despite the lack of significant reduction in spasticity, 87.5% of participants noted reduction in spasms, 73.3% reported pain reduction, and 73.3% had reduced stiffness. Follow-up of the subjects ranged from 8 to 20 months, and the authors reported that most subjects were still using TENS units on an as needed basis.
The use of TENS units is recommended in the skilled rehabilitation setting under the guidance of a trained therapist. If the patient demonstrated clinical improvement, recommendation for purchase is appropriate and indicated.
There is some evidence to suggest that functional electrical stimulation may result in a reduction of spasticity. Szecsi et al (50) found a significant short-term reduction in spasticity in patients with MS who engaged in functional electrical stimulation-assisted cycling for 2 weeks. In addition, there are currently two functional neuromuscular electrical stimulation devices, the BioNess L300 and the Walkaide, that are being used clinically to manage MS-related foot drops. Although there has been significant marketing and media hype regarding the efficacy of these devices on safety, gait quality and speed, ambulation distance, and overall strengthening, there is currently a paucity of research documenting the manufacturers’ claims.
Orthotics. Various bracing devices are frequently prescribed to assist patients with weakness or joint instability secondary to spasticity. There are conflicting reports on the benefit of orthotics such as the hip flexion assist orthosis, static ankle/foot orthosis, and dynamic ankle/foot orthosis in terms of improved ambulation (51–53). It is postulated that the use of assistive devices such as orthotics may reduce energy expenditure by assisting mobility and improving balance. However, there are no published studies on the effects of orthotics in reducing spasticity.
Serial casting. This modality is utilized to correct deformity, lengthen contractures, and reduce spasticity through the repeated application and removal of casts (54). The precise mechanism of serial casting is unknown. Muscles immobilized in a shortened state usually lose sarcomeres. It has been theorized that serial casting may reduce excitation of the alpha or gamma motor neurons in the spinal cord and increase the length and number of sarcomeres in the targeted muscle (55,56). Serial casting is noninvasive and strong enough to counteract joints with increased tone that may not be amenable to dynamic casts. In addition, serial casting can be done as an adjunctive agent to oral agents, as well as nerve blocks. The disadvantage of casting is the time required to apply and change the cast. It can be difficult for caregivers, as the cast cannot get wet and is heavy, making assistance with transferring challenging. Skin ulcers or breakdown are uncommon but can occur with improperly placed casts. Contraindications to casting include open wounds, unhealed fractures, impaired sensation or circulation, and uncontrolled hypertension.
Oral Medications
Oral agents are frequently used in the management of spasticity for people with MS. The choice of the most appropriate agent is dependent on the characteristics of the person and his or her muscle overactivity, distribution (focal vs diffuse), time of presentation (all day vs nighttime), presence of other medical conditions, and cognitive status. There are also factors that are agent-specific, such as the side-effect profile and cost of each agent. Three agents are approved for use in the treatment of spasticity: baclofen, dantrolene, and tizanidine (57). Although only these agents have an approval for the management of spasticity, there are several other oral medications that are commonly used. It has been reported that most patients with MS are inadequately treated with oral medications due to clinicians’ concerns about sedation and fatigue (6). The discussion that follows contains a summary of the agents most commonly used in this population.
Baclofen. Baclofen is believed to mediate its activity at the gamma-aminobutyric acid (GABA) receptors. Through its binding to GABA-B receptors, it reduces spasticity, specifically flexor-type spasticity. Baclofen is well tolerated in the MS patient population and is dosed between 5 and 120 mg daily (58–61). It is a common first-line oral agent for spasticity management. Renal, hepatic, and hematopoietic safety has been demonstrated in patients with MS taking the medication for more than 3 years (62). Treatment is more effective when begun at earlier stages of spasticity. Typically, initial dosing is recommended at night due to the potential for fatigue or sedation. Titration may be gradually increased to TID or QID dosing. Common side effects include somnolence, fatigue, constipation, nausea, and vomiting. Liver function testing is recommended every 6 months.
Dantrolene sodium. Dantrolene inhibits calcium release from the sarcoplasmic reticulum and thereby reduces the strength of muscle contraction (63). The recommended dosage is 25 to 400 mg daily with a slow titration. Doses higher than this can result in severe hepatotoxicity (64). The half-life of dantrolene is approximately 15 hours. Although dantrolene had reduced muscle tone and increased ROM, its most profound effect is reduction in clonus. Because of its effect at the skeletal muscle level, it usually does not affect cognitive functioning. However, because of dantrolene’s site of action at the skeletal muscle level, one of its main side effects is muscle weakness. This effect severely limits its use in the MS patient population (65,66).
Tizanidine. Tizanidine modulates the release of glycine and excitatory neurotransmitters and is an alpha-2 central agonist (64). Several studies investigated its efficacy in the management of spasticity for people with MS (67–70). It is available in capsule and tablet formulations. It is recommended to begin dosing at 2 mg and gradually titrate up to 36 mg daily as tolerated. Comparative studies between tizanidine, baclofen, and dantrolene demonstrate increased to equivalent efficacy with tizanidine and baclofen. The side-effect profile for tizanidine includes sedation, dizziness, dry mouth, and asthenia, which has limited its usefulness in this population due to poor tolerability. It is suggested that liver function testing be monitored every 3 to 6 months to ensure patient safety. Hepatic transaminase levels of three times the upper limit of normal have been reported in 5% of patients who participated in postmarketing surveillance studies (57). Patients are typically asymptomatic, and a return to normal transaminase levels usually occurs after withdrawal of tizanidine therapy.
Benzodiazepines. Diazepam and clonazepam are commonly used for spasticity management in patients with MS. Diazepam is a GABA-A agonist, and animal studies have found that it increases presynaptic inhibition of polysynaptic and monosynaptic reflexes (71). Past work has demonstrated the efficacy of diazepam in the management of spasticity associated with MS (72–74). The recommended dosage for diazepam is between 4 and 40 mg daily and should be given in two to four divided doses. The side-effect profile is significant and includes sedation and memory impairment. Although comparative studies between diazepam, dantrolene sodium, and baclofen showed no significant difference in therapeutic effects, diazepam’s side-effect profile has limited patient tolerance. Overdosage can lead to somnolence, coma, and death, and abrupt withdrawal can lead to insomnia, anxiety, seizures, psychosis, and even death (64).
When compared with baclofen, clonazepam has demonstrated equivalent clinical efficacy in the management of spasticity overall and superior efficacy with spasticity secondary to cerebral origin (75). It is frequently used to treat phasic limb spasticity in people with MS.
Cannabis. Cannabis has been used as an agent to treat spasticity and pain that is found in patients with MS. It also demonstrated reduced central nervous system neurodegeneration in animal models of MS (76). The active component of marijuana is 9-tetrahydrocannibinol (9-THC) (77), and it is believed that most of the effects of cannabinoids occur through binding to CB1 and CB2 receptors. CB1 receptors are present throughout the nervous system, and CB2 receptors are located mainly on immune cells (64,78,79).
Cannabis is available as synthetic THC agents in two tablet forms, dronabinol and nabilone. Both agents are approved for chemotherapy-induced nausea, human immunodeficiency virus-related wasting, and glaucoma (64). Dronabinol is marketed under the trade name Marinol® and is available in 2.5-, 5-, and 10-mg capsules, with a maximum daily dosage of 20 mg. Nabilone is marketed under the name Cesa-met®. It is available in 1-mg capsules with a recommended maximum dose of 6 mg per 24 hours. Sativex® is a 9-THC containing oral-mucosal spray currently available in Canada, where it is approved for the treatment of MS-related pain (80).
The literature concerning the use of cannabis on MS-related spasticity has been mixed. Zajicek et al (78) conducted a randomized, double-blind, placebo-controlled trial evaluating the effects of cannabinoids on spasticity in 611 patients and found that the use of cannabis did not result in significant change in spasticity scores. They did note subjective improvement in spasm and sleep quality as well as a decrease in hospitalizations for exacerbation in the treatment group. The authors hypothesized that their inability to detect changes in spasticity may have been due to the inadequacy of the metric used (AS) to detect subtle changes. In addition, the patient sample consisted of a very impaired population as demonstrated by the fact that the EDSS of almost all the participants was greater than 6.0. It is possible that similar to the other oral antispasmodic medications, a greater benefit occurs when interventions are implemented earlier in the MS disease course. A 12-month follow-up study was conducted on the same patient group (81). Eighty percent of the original participants were followed up and they remained on active or placebo treatment. No significant improvement in spasticity scores was noticed in the active or placebo group. Another randomized, double-blind, placebo-controlled trial of 160 patients with MS demonstrated statistically significant reduction of spasticity with active cannabis compound over placebo (82). Both studies reported good tolerance of the medication. Common reported side effects of oral THC included increased appetite, dry mouth, somnolence, and bowel disturbance.
Corey-Bloom et al conducted a randomized, placebo-controlled trial with 30 MS patients experiencing spasticity. Treatment with inhaled cannabis resulted in reduced scores on the MAS by an average of 2.74 points versus placebo. Additionally, cannabis treatment resulted in reduction of pain scores on the VAS by an average of 5.28 points. There were no significant adverse events from treatment (83). Novotna et al published a randomized, double-blind, placebo-controlled study of nabixomols (Sativex), oromucosal THC/CBD spray, as an adjunct therapy for refractory spasticity in MS. Inclusion criteria included moderate-level spasticity (NRS spasticity severity score greater than or equal to 4) of at least 3 months duration that is not relieved with antispasticity medications (84). Inhaled cannabis is not currently available in the United States.
The MOVE 2 study treated spastic MS patients with THC/CBD spray and assessed their response at months 1 and 3. Forty-two percent of patients evaluated at month 1 showed greater than 20% NRS reduction and 40% of those evaluated at month 3 revealed greater than 30% improvement in the NRS score. The average modified Ashworth score at 1 month fell from 3 to 2.7 and maintained at 2.6 after 3 months. An extension study revealed 53% had a greater than 20% NRS reduction at 1 year and 41% had a greater than 30% reduction (85).
The potential clinical benefit of cannabis for people with MS remains unclear. In a survey of 14 patients with MS, each subject was asked a series of questions regarding the perceived benefit of marijuana (86). The participants reported improvement and reduction in spasms, pain, tremors, nausea, numbness, and bladder and bowel symptoms. Some negative effects reported by patients included decreased ability to concentrate as well as ataxia and fatigue. All participants used medical marijuana. Smoking marijuana for medical purposes is currently legal in 23 states; however, the federal government’s authority can override a state’s position.
Other oral medications. Threonine is a naturally occurring amino acid that is believed to have effects on the motor reflex arc and was reported to increase glycine levels in animal studies (87). Glycine is released from the interneurons in the gray matter of the spinal cord and Renshaw cells and is believed to inhibit excessive motor reflexes. Threonine is believed to further enhance glycinergic postsynaptic inhibition and thereby reduce increased motor activity or spasticity. A double-blind, placebo-controlled crossover trial in 26 ambulatory patients with MS was conducted to evaluate the effects of 7.5 mg/d of threonine on objective and subjective measures of spasticity (87). Threonine failed to demonstrate improvement in Ashworth scores or in the Patient Spasticity Scale or elevated glycine levels despite demonstration of elevated threonine levels in the cerebrospinal fluid and plasma. There was also no improvement in neurophysiologic studies. Another double-blind, placebo-controlled crossover study in patients with MS and SCI showed modest benefit in AS spasticity scores (88). This study by Lee et al suggested greater benefit in patients with spinal cord involvement. Both studies reported little if any sedation or confusion as side effects during the administration of threonine.
Gabapentin is approved for use as an anticonvulsant for partial seizures and for treatment of postherpetic neuralgia; however, its mechanism of action for spasticity is unknown (89). The literature is limited, with Dunevsky and Perel (90) reporting on its effectiveness on the spasticity of two people with MS-related spasticity. A double-blind, placebo-controlled trial evaluated the utility of gabapentin as a primary and an adjunctive treatment for the management of spasticity (89). Gabapentin was noted to be superior as an adjunctive agent than as a primary antispasmodic therapy. It is generally well tolerated but can cause somnolence and fatigue in some patients and required no laboratory monitoring for safety.
Cyproheptadine is another drug that has been studied for its antispasmodic effects in this population (91). It is also indicated for the treatment of allergic symptoms as well as appetite stimulation (64). Interestingly, it has also been studied as a treatment for baclofen withdrawal symptoms (92). It functions as a serotonergic antagonist that modulates spinal reflexes to reduce spasticity. The recommended dosing is 4 mg QHS with gradual titration to 16 mg. Doses of 12 to 24 mg of cyproheptadine were studied in people with or SCI-related spasticity over 4 to 24 months and found effective as a primary and adjunctive antispasmodic agent (91).
Another drug studied for its antispasmodic effects is progabide, a GABA-A and GABA-B agonist (93). A randomized, double-blind, placebo-controlled crossover trial was conducted with this agent but failed to demonstrate functional changes. There were also issues with tolerability due to its side effects of drowsiness, dizziness, and nausea. In addition, elevation of transaminase levels required discontinuations in some people.
Carisoprodol, a derivative of the muscle relaxant meprobamate, is used for the treatment of skeletal muscle pain and frequently used to treat low back pain and fibromyalgia (27). Meprobamate functions through GABAergic neurotransmitter pathways and inhibits polysynaptic spinal reflexes. Carisoprodol was beneficial in patients with MS at 350 mg, BID. Its overall tolerability was good, with drowsiness and confusion as the main side effects.
All of the aforementioned agents can be used alone or in combination with each other as well as in combination with other modalities including therapy, chemodenervation, ITB pump, and surgery. Gradual titration is recommended for all of the antispasmodic medications to minimize severity of side effects. Conversely, when discontinuing an agent, gradual titration is also recommended, as abruptly stopping the medication can trigger the onset of seizures and withdrawal symptoms.
Chemical Denervation
Spasticity that is focal or segmental can be effectively managed with chemical denervation (CD). Many of the systemic treatments for MS can cause sedation and fatigue confusion or negatively affect cognition. One of the biggest advantages of CD over oral medications is the lack of these side effects, which may make it much more appealing to patients. Unfortunately, none of the agents is a permanent remedy, and people will have to return for reinjection. CD is used to alleviate spasticity or flexor synergy patterns caused by UMN lesions. It can also be used before orthopedic procedures to determine if a joint has intra-articular contracture. If no improvement in spasticity or ROM is achieved through CD, then surgical intervention is usually recommended. As previously stated, spasticity can lead to inactivity, decreased joint ROM, and possibly contractures. Contractures can lead to skin breakdown and increased difficulty for the individual or caregiver to perform ADL. Another benefit of CD is ease in caregiving and reduced risk of contracture, ulcer, and inactivity. Agents commonly used for CD include lidocaine, bupivacaine, phenol, and botulinum toxin (BoNT).
Phenol/ethanol/alcohol. The first use of phenol for the treatment of spasticity was in 1965 by Nathan et al (94). Concentrations of 5% to 7% of phenol are recommended for optimal effect and minimal side effects. Concentrations lower than 5% are usually ineffective, whereas concentrations greater than 7% can cause significant side effects including dysesthias, fibrosis, cellular damage, and cardiovascular complications. Phenol mixed with glycerin will form a more viscous solution that can reduce medication spread and minimize nerve injury and side effects (95). Phenol is indicated in patients with upper and lower limb spasticity who have demonstrated little to no functional benefit from other interventions including therapy, oral medications, and possibly BoNT injections. The effect of ethanol on spasticity in patients with MS is unknown. Phenol treatment has demonstrated improved personal hygiene care, transfers, ADL management, and decrease in skin ulcer formation in patients with MS (96,97). It has also resulted in improved gait pattern with decreased scissoring in patients who underwent obturator nerve blocks (98).
Ethanol concentrations between 45% and 100% are recommended for CD. It has demonstrated benefit in poststroke knee flexor spasticity, elbow flexor spasticity, ankle spasticity, and adductor spasticity secondary to stroke or TBI (99–103). The advantages of both phenol and ethanol include immediate onset of action, duration of action spanning 3 to 9 months, and low cost. Both phenol and ethanol are indicated for relief of spasticity in ambulatory and bedbound patients.
The potential adverse effects of phenol and ethanol injections include paresthesias, dysesthesias, and pain and are more likely if sensory fibers are affected. Peripheral edema and muscle necrosis are also possible.
Botulinum toxin (BoNT). There are seven immunological types of BoNT available, with only types A and B approved for clinical use in the United States (104). Although all seven serotypes work through inhibition of acetylcholine release from the neuromuscular junction, they differ in their specific site of action. Please refer to earlier chapters on specific subtype pharmacology. The toxin works selectively at peripheral cholinergic endings that lead to binding, internalization, and toxin activation at the neuromuscular junction resulting in CD. The inhibition of acetylcholine release causes muscle weakness. The effects of the toxin last approximately 60 to 90 days, depending on the serotype injected (105).
There are four botulinum products approved for use in the United States.
• Type A
![]() Onabotulinum toxin (BOTOX®, BOTOX Cosmetic®)
Onabotulinum toxin (BOTOX®, BOTOX Cosmetic®)
– BOTOX—cervical dystonia, severe primary axillary hyperhidrosis, strabismus, blepharospasm, neurogenic detrusor overactivity, chronic migraine, upper limb spasticity
– BOTOX Cosmetic—moderate to severe glabellar lines, moderate to severe lateral canthal lines
![]() Abobotulinum toxin (Dysport®)—cervical dystonia, moderate to severe glabellar lines
Abobotulinum toxin (Dysport®)—cervical dystonia, moderate to severe glabellar lines
![]() Incobotulinum toxin (Xeomin®)—cervical dystonia, blepharospasm, moderate to severe glabellar lines
Incobotulinum toxin (Xeomin®)—cervical dystonia, blepharospasm, moderate to severe glabellar lines
• Type B
![]() Rimabotulinum toxin (MyoBloc®)—cervical dystonia
Rimabotulinum toxin (MyoBloc®)—cervical dystonia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






