CHAPTER 23
Spasticity Due to Disease of the Spinal Cord: Pathophysiology, Epidemiology, and Treatment
Heather W. Walker, Alice J. Hon, and Steven Kirshblum
Spasticity is a common sensorimotor symptom complex commonly experienced by individuals sustaining spinal cord injury (SCI) with upper motor neuron (UMN) involvement, that is, injury above the level of the conus medullaris. Although spasticity may occasionally contribute to improved function (ie, transfers, standing, ambulation, and assisting in activities of daily living [ADL]), it more often leads to various complications including contractures, pain, impaired function, and decreased quality of life (QOL). This chapter discusses the presumed pathophysiology, causes, classification, and treatment options of this common problem in regard to a person with SCI.
DEFINITION AND SCOPE OF THE PROBLEM
Lance (1) classically described spasticity as “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome” (UMNS). Others have proposed newer definitions to include the many different clinical signs and symptoms of spasticity (2–5). Spasticity is a component of the UMNS that is composed of positive and negative symptoms. The positive symptoms include hyperreflexia, clonus, spasms, and postural abnormalities, and the negative symptoms include weakness, incoordination, fatigue, and pain (6). The positive symptoms are easier to see and treat, whereas the negative symptoms are more functionally limiting and may be more resistant to treatment. The components of spasticity may be further delineated into tonic and phasic spasticity; tonic spasticity manifests clinically as increased tone that is due to an exaggeration of the tonic component of the stretch reflex, whereas phasic spasticity can be observed clinically as hyperreflexia and clonus due to an exaggeration of the phasic component of the stretch reflex (2).
Spasticity is a common complication of SCI. The incidence in UMN-related SCI during rehabilitation is approximately 70%, with roughly half the patients requiring pharmacologic intervention (7,8). At 1 year postdischarge, 78% of people have spasticity, with 49% requiring pharmacologic treatment (7). Spasticity occurs more frequently in persons with cervical and upper thoracic SCI than in those with lower thoracic and lumbosacral SCI and is usually more significant in persons with specific types of incomplete injuries, with persons with ASIA Impairment Scale (AIS) grades B and C having greater issues with spasticity than persons with grades A or D (7,9). More recently, Sköld (10) published that patients with incomplete SCI reported a higher incidence of self-reported spasticity and possibly greater variability throughout the day as compared with individuals with complete SCI. This group also investigated the relationship between self-reported and objective evidence of spasticity and found that in patients reporting the presence of spasticity, only 60% had measurable spasticity on physical examination.
Although each of the components of the UMNS may have an impact on an individual’s function (eg, fatigue, incoordination, cocontraction of antagonist muscles), QOL, pain, and other aspects of his or her life, this chapter focuses only on spasticity as one of the components of the UMNS.
PATHOPHYSIOLOGY
Immediately after SCI, there are depressed spinal reflexes during the state of spinal shock, followed by development of hyperreflexia and spasticity over the following weeks to months. The pathophysiology of spasticity is not completely understood; however, it is believed to arise primarily from the loss of the effect of numerous descending inhibitory pathways. These include reciprocal Ia interneuronal inhibition, presynaptic inhibition, Renshaw-mediated recurrent inhibition, group II afferent inhibition, and the Golgi tendon organs (11). Axonal collateral sprouting and denervation supersensitivity are changes that may also play a role in the development of spasticity (12). In addition to these changes that result in decreased input from descending inhibitory pathways, mechanical changes to the muscle after SCI may also play a role in the pathophysiology of spasticity (13). These mechanisms are further explored individually.
NORMAL MOTOR CONTROL
To understand how loss of descending inhibition plays a role in spasticity, one must first understand the physiology of the monosynaptic stretch reflex arc. The muscle spindles are specialized intrafusal fibers that, when stretched, send afferent impulses to the spinal cord by way of type Ia and type II afferent fibers, providing information about muscle length and position. Once activated, the Ia fibers make a monosynaptic connection with, and have an excitatory influence over, the alpha motor neurons supplying the extra fusal muscle fibers of both the agonist muscles and the agonist’s synergistic muscles, leading to contraction of these muscle groups. The Ia fibers also synapse on interneurons that inhibit antagonist muscle groups, thereby preventing contraction of these muscles during activation of the agonist muscle groups; this inhibitory pathway is referred to as reciprocal Ia inhibition and can be altered after SCI. Clinically, reciprocal inhibition can be grossly observed by eliciting monosynaptic muscle stretch reflexes: when the tendon is tapped, a stretch is applied to the target muscle, which is transmitted to the spinal cord through the Ia afferent fibers. The Ia afferent fibers exert an excitatory influence over the efferent alpha motor neurons that innervate the target muscle and an inhibitory influence to the interneurons that synapse on the alpha motor neurons that innervate the antagonist muscles. This reciprocal Ia inhibition allows contraction of the target muscle while inhibiting contraction of the antagonist muscles. Impairment of reciprocal inhibition after SCI may result in simultaneous coactivation of agonist and antagonist muscle groups, as is often seen in patients with spasticity (14).
Recurrent inhibition is mediated by Renshaw cells, which are inhibitory interneurons located in the ventral horn of the spinal cord. Axon collaterals from alpha motor neurons synapse on and activate the Renshaw cells, which in turn project inhibitory impulses back to these motor neurons, as well as to Ia inhibitory interneurons. Renshaw activity decreases the activity of the motor neurons that were previously active and also inhibits the Ia inhibitory interneurons. The level of recurrent inhibition has been explored in patients with UMN lesions, and these individuals have been noted to maintain normal recurrent inhibition at rest, but impaired recurrent inhibition during voluntary movement; this may contribute to impaired motor function in these patients (12,15). There is evidence for increased recurrent inhibition in the SCI population, which increases inhibition to the Ia interneurons (16). This ultimately allows for co-contraction of agonist and antagonist muscle groups due to the decreased Ia interneuron activity.
Reduction in presynaptic inhibition of Ia afferents is another potential contributor to the pathophysiology of spasticity in SCI. Reciprocal inhibition was described by Sherrington in 1906, and this process is responsible for relaxation of an antagonist muscle during contraction of the agonist (17). In the absence of reciprocal inhibition, cocontraction of agonist and antagonist muscle groups is seen simultaneously, interfering with intentional voluntary movement. Gamma-aminobutyric acid (GABA) mediates spinal inhibition both presynaptically and postsynaptically. Presynaptic inhibition of Ia afferents occurs when the inhibitory amino acid GABA binds to receptors on the Ia terminals, which subsequently increases the amount of input required to activate the alpha motor neurons (18). The decreased excitatory input to the alpha motor neurons in turn depresses the monosynaptic stretch reflex. Postsynaptic activation of GABA-A receptors can decrease the activity of motor neurons and interneurons (18). After SCI, the decrease in presynaptic inhibition ultimately results in increased activity of the alpha motor neuron; this may contribute to the hyperreflexia and spasticity seen in these individuals (12). It is possible to modulate the presynaptic inhibition in individuals with SCI with the use of GABA-ergic medications including baclofen and diazepam, discussed later in this chapter.
Nonreciprocal Ib inhibition is another mechanism that may play a role in the development of spasticity of supraspinal origin but does not appear to be involved in spasticity related to SCI. Golgi tendon organs, which are contraction-sensitive receptors, have group I afferents and Ib inhibitory interneurons that project to the spinal cord and are involved in preventing antagonist muscles from firing while the agonist is firing (19). There is evidence for replacement of Ib inhibition with facilitation in hemiplegic individuals with supraspinal lesions, leading to simultaneous cofiring of agonist and antagonist muscle groups (20); however, studies in individuals with SCI have shown that Ib inhibition is unaltered (Figure 23.1).
Two additional mechanisms that may play a role in the development of spasticity after SCI are axonal sprouting and denervation supersensitivity. Ditunno et al (21) describe the transition from spinal shock immediately after SCI to the development of spasticity and hyperreflexia 1 to 12 months later. In their proposed four-phase model of spinal shock, there is observation of areflexia or hyporeflexia, as well as paralysis and muscle flaccidity for the initial 0 to 24 hours postinjury. These findings are due to the loss of excitatory input from supraspinal pathways, including vestibulospinal and reticulospinal pathways, among others. The loss of descending inhibitory input to spinal inhibitory interneurons may cause further hyporeflexia. In the second phase of spinal shock, there is return of the tibial H reflex 1 to 3 days after injury, although muscle stretch reflexes are still absent. This is likely due to denervation supersensitivity, which causes increased neuronal firing in response to neurotransmitters and has been reported to occur in the brain and spinal cord. The denervation supersensitivity may be due to decreased reuptake of excitatory neurotransmitters, upregulation of receptors on the postsynaptic membrane, or alteration of degradation and synthesis of receptors. Phases 3 and 4 of Ditunno’s model describe early hyperreflexia and later development of spasticity in patients with SCI. The proposed physiologic mechanism for both phases is axonal regrowth. New synapses are formed by spinal afferents and interneurons, as well as spared supraspinal descending pathways (23). Axonal sprouting of spared descending motor tracts may result in motor recovery, whereas axonal sprouting of the neurons involved in segmental reflexes may produce less desirable effects, such as the development of hyperreflexia and spasticity (24).
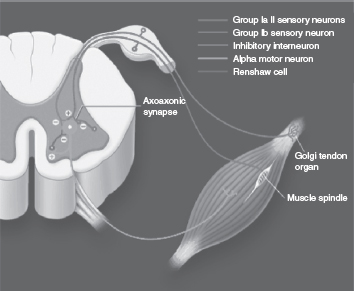
FIGURE 23.1 Potential spinal mechanisms involved in the development of spasticity.
Source: Adapted from Ref. (22). Satkunam LE. Rehabilitation medicine: 3. Management of adult spasticity. CMAJ. 2003;169:1175.
Intrinsic changes within the muscle may also play a role in the development of increased muscle tone. These mechanical changes may include loss of sarcomeres, increased stiffness of muscle fibers, altered muscle fiber size and distribution of fiber types, and changes in collagen tissue and tendons (25,26). The work of Kamper et al (27) in stroke patients demonstrated that muscle fibers played some part in the phenomenon of spasticity as decreasing the initial length of tested spastic metacarpophalangeal fibers reduced muscle stiffness suggesting that the biomechanical qualities of muscle fibers play some part in the development of spasticity. These changes in spastic muscles may be a result of the development of subclinical contracture rather than true reflex hyperexcitability (26) or may be an intrinsic property of the changes in biomechanical properties of the muscle (27).
SPASTICITY MEASURES
Although spasticity may seem simple to recognize clinically, it is difficult to quantify. Having tools available that objectively measure spasticity is important to evaluate and monitor response to treatment. Currently, there are a variety of different outcome measures available that can be used to quantify tone (28). These outcome measures can be categorized into subjective measures including clinical scales and self-reported measures, and objective measures including biomechanical techniques and electrophysiologic measurements. Extensive reviews have been published on the various subjective and objective spasticity measures (29–31); however, we discuss the tools that are most commonly used to assess spasticity in individuals with SCI. A review of measures applicable in the clinical setting for persons with SCI has been undertaken that revealed 66 different measures found (5) of which only six had been tested psychometrically (32). It is clear that no single outcome measure fully captures the multidimensional nature of spasticity.
Clinical Measures
Ashworth Scale and Modified Ashworth Scale. The Ashworth Scale (AS [33]; developed initially to assess hypertonicity in persons with multiple sclerosis [MS]) and the Modified Ashworth Scale (MAS [34]; Table 23.1) are the most common clinical methods used to measure the degree of muscle tone or “the sensation of resistance felt as one manipulates a joint through a range of motion (ROM), with the subject attempting to relax” (35). These tests are relatively quick and simple to perform and do not require any instrumentation. When performing these tests, the examiner passively moves the subject’s joints through full ROM and judges the degree of tone felt during the passive range on a 0-to-4 scale on the AS, with a score of 0 indicating that there is no increase in tone, and a score of 4 signifying that the affected part is rigid in flexion or extension. For the MAS, an additional grade was added (1+) to enhance sensitivity at the lower end of the scale. The amount of time allotted for passive movement of the joint through full ROM is not well specified, although several studies suggest 1 s. In regard to the testing procedure, Pandyan et al (36) suggest that the examiner should limit the number of repetitions performed during the testing procedure because repetitive passive ROM will decrease the resistance of the muscle to passive stretch and may affect scoring on the AS and MAS. Sköld (37) likewise report a decrease in spasticity with repetitive passive movements.
TABLE 23.1
CLINICAL MEASURES OF SPASTICITY | |
Ashworth Scale (AS) | |
0 | No increased tone |
1 | Slight increase in tone, giving a “catch” when the affected part is moved in flexion or extension |
2 | More marked increase in tone, but the affected part easily flexed |
3 | Considerable increase in tone; passive movement difficult |
4 | Affected part rigid in flexion or extension |
Modified AS | |
0 | No increased tone |
1 | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the ROM when the affected part(s) is moved in flexion or extension |
1+ | Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM |
2 | More marked increase in tone, but the affected part easily flexed |
3 | Considerable increase in tone; passive movement difficult |
4 | Affected part rigid in flexion or extension |
Changes compared to the standard AS are italicized.
ROM, range of motion.
A number of investigators have studied the interrater reliability of these scales when used to evaluate people with SCI. Haas et al (38) found that the most commonly used scores were generally on the lower end of the scales, and interrater reliability was variable among muscle groups tested for both scales. Sköld (37) investigated the relationship between the objective findings of spasticity rated using the MAS and patients’ self-reported ratings of spasticity using a Visual Analog Scale and found a significant correlation between these two measures. Lechner et al (39), however, reported a weak correlation between self-reported spasticity rated on the Visual Analog Scale and clinical measures of spasticity according to the AS in three of eight subjects with spasticity secondary to SCI and no correlation in the remaining five subjects.
Spasm Frequency Scales. Quantification of the frequency of spasm occurrence has also been described and is often used clinically. One of the most commonly used scales is the Penn Spasm Frequency Scale (PSFS), which was created to measure the effectiveness of intrathecal baclofen (ITB) in the treatment of spasticity in subjects with spasticity of spinal origin (Table 23.2). This scale measures the number of spasms experienced by patients within a 1-hour period. The PSFS measures an entity different from tone, as Priebe et al (41) found no correlation between it and the AS. The PSFS was modified by Priebe et al (41) and is referred to as the modified PSFS. This consists of a second self-report 3-point scale only if the PSFS is greater than 1 and assesses severity of spasms from “1 = mild” to “3 = severe”; this provides a more comprehensive understanding of the individual’s spasticity status. An alternative scale is the Spasm Frequency Score, which measures the number of spasms per day (Table 23.2[42]).
TABLE 23.2
SPASM FREQUENCY SCALES | |
Penn Spasm Frequency Score (PSFS) | |
0 | No spasms |
1 | Mild spasms induced by stimulation |
2 | Infrequent spasms occurring less than once per hour |
3 | Spasms occurring more than once per hour |
4 | Spasms occurring more than 10 times per hour |
Modified PSFS: Two Part | |
Part 1: Spasm frequency score (as mentioned previously) | |
Part 2: Spasm severity scale | |
1 | Mild |
2 | Moderate |
3 | Severe |
Spasm Frequency Score | |
0 | No spasms |
1 | One or fewer spasms per day |
2 | Between 1 and 5 spasms per day |
3 | Five to more than 10 spasms per day |
4 | Ten or more spasms per day, or continuous contraction |
Spinal Cord Assessment Tool for Spasticity. This scale was developed by Benz et al (3) to measure spasticity in SCI. This easy-to-administer tool utilizes elements of the standard neurologic examination of the lower extremities (LE). The Spinal Cord Assessment Tool for Spasticity (SCATS) flexor spasms and clonus scores correlate well with AS scores, but only the SCATS clonus score correlates with the PSFS. This tool may provide additional information in comparison to the AS and MAS in assessing multi-joint spasticity (Table 23.3).
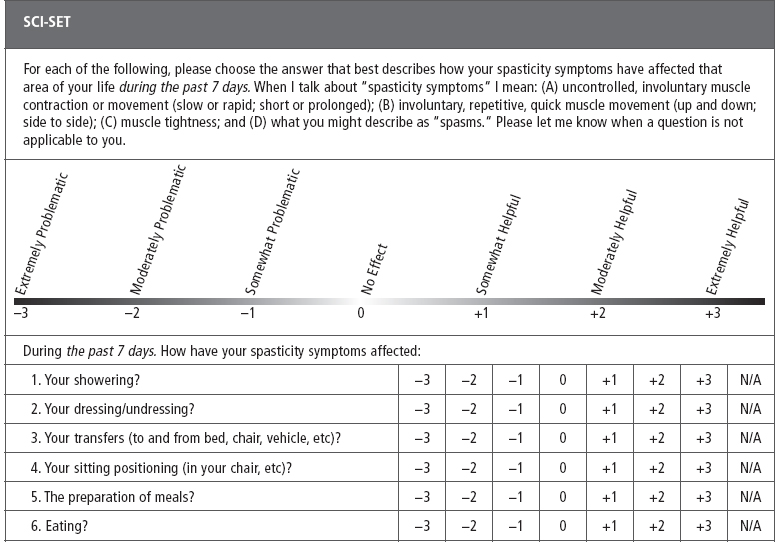
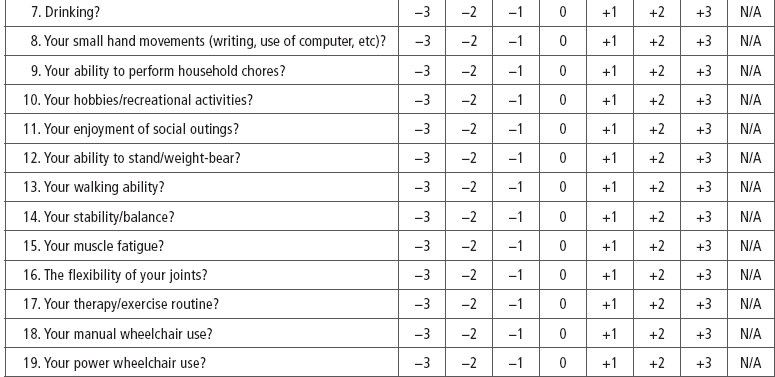
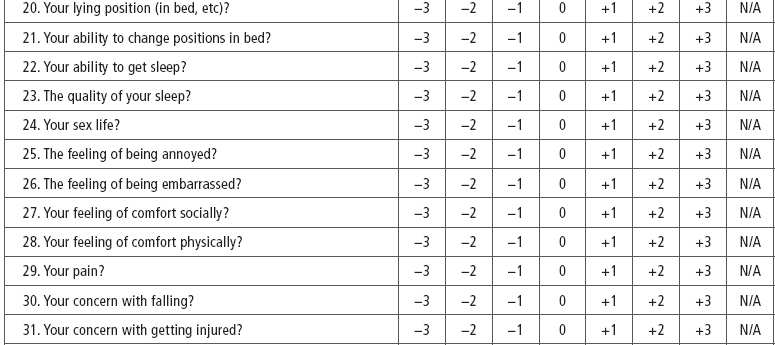
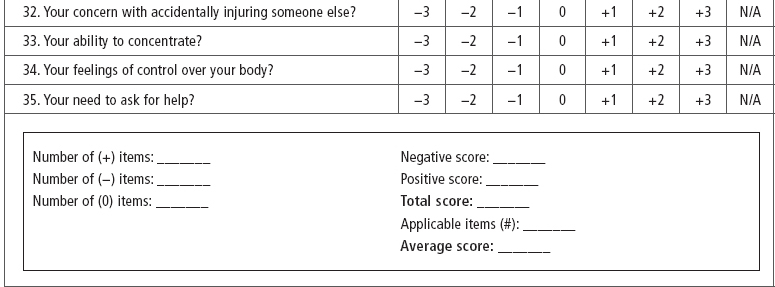
SCI-Spasticity Evaluation Tool (SCI-SET). Described by Adams et al (43) the SCI-SET is a 35-item, 7-day recall self-report scale. This tool assesses the impact of spasticity on various ADL to issues of social participation, with a Likert scale of −3 (extremely problematic) to +3 (extremely helpful) in asking how spasticity affects patients’ lives. It is a short survey that has shown test–retest reliability and construct validity (Table 23.4).
TABLE 23.3
SPINAL CORD ASSESSMENT TOOL FOR SPASTIC REFLEXES | |
SCATS: Clonus | |
Clonus quantified in response to rapid dorsiflexion of the ankle | |
0 | No reaction |
1 | Mild, clonus maintained less than 3 seconds |
2 | Moderate, clonus persists 3–10 seconds |
3 | Severe, clonus persists more than 10 seconds |
SCATS: Flexor Spasms | |
Measurement of excursion of big toe into extension, ankle dorsiflexion, knee flexion, or hip flexion when pinprick stimulus applied to plantar surface of the foot | |
0 | No reaction to stimulus |
1 | Mild, less than 10° |
2 | Moderate, 10°–30° |
3 | Severe, greater than or equal to 30° |
SCATS: Extensor Spasms | |
Starting position with hip and knee placed at 90°–110° of flexion with contralateral limb extended. Hip and knee joints then simultaneously extended and duration of quadriceps muscle contraction is measured | |
0 | No reaction |
1 | Mild, contraction maintained less than 3 seconds |
2 | Moderate, contraction persists 3–10 seconds |
3 | Severe, contraction persists more than 10 seconds |
Source: Summarized from Ref. (3). Benz EN, Hornby TG, Bode RK, et al. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86:52–59.
Patient Reported Impact of Spasticity Measure. The Patient Reported Impact of Spasticity Measure is an instrument that standardizes the collection of self-report information (both positive and negative impacts) relevant to the clinical assessment of spasticity (44). There are subscales that include social avoidance/anxiety, psychological agitation, daily activities, need for assistance/positioning, need for intervention, and social embarrassment from the spasticity.
TABLE 23.4
Tardieu Scale. The Tardieu Scale was first introduced in 1954 and has since undergone revisions. Although this scale has mostly been used in cerebral palsy and adult stroke, it has also been used in SCI and has some benefits over other scales. The key to this scale is that resistance to passive stretch may be from more than just spasticity (ie, soft tissue changes) and that there are “neural versus peripheral contributions” to the spasticity present. There are three key components to the testing: (a) velocity of stretch, (b) quality of muscle reaction, and (c) angle of muscle reaction. This can be used in SCI when serial casting is performed (see later). By varying the velocity of the stretch during testing, the Tardieu may be superior to the Ashworth in the assessment of true spasticity, which by definition has a velocity component.
Biomechanical Measures
Biomechanical techniques can be used to objectively quantify spasticity by evaluating resistance to passive movement at a joint. Commonly used techniques are the pendulum test and measurements using isokinetic dynamometers (45–49). The pendulum test was first described by Wartenberg (47) in 1951 and assesses spasticity in the hamstring and quadriceps muscles. To perform this test, the patient is in the seated or supine position with the examination table ending at the distal thigh of both legs; this allows passive flexion and extension at the knee joint without interference of movement from the examination table. The patient is asked to relax the lower limb, and the examiner passively extends the knee fully, then releases, allowing the limb to fall freely. Electrogoniometers may be used to evaluate the swing of the leg at the knee joint, and tachometers can assess the rate of movement. In the spastic limb, the degree of movement at the knee joint is decreased when compared with movement in the nonspastic limb. It is difficult to determine whether the dampening of movement is due to intrinsic changes within the spastic muscle, such as alterations in viscoelastic properties, or due to the other pathophysiologic changes after SCI that result in velocity-dependent resistance to movement (50). Although the pendulum test has its limitations, the test–retest variability and reliability have been evaluated, and this test has been shown to have a high correlation between measures on repeated trials using a commercially available isokinetic dynamometer (46).
Electrophysiologic Measurements
There are a variety of electrophysiologic methods that can be used to objectively assess spasticity; however, these techniques are used primarily for research purposes. To understand the most commonly used methods, one must have a basic understanding of several electrophysiologic tests. Maximal electrical stimulation of a peripheral nerve results in the development of a compound motor action potential (CMAP), which can be recorded over a muscle innervated by the stimulated nerve; this is the M response. The Hoffman reflex, or H reflex, is a late response that can be obtained by delivering a submaximal electrical stimulus to the tibial nerve with the CMAP recorded over the soleus. The H reflex is an electrically elicited reflex comprised of an orthodromic sensory and an orthodromic motor response. The stimulus delivered to the tibial nerve travels up the large Ia afferents into the spinal cord, synapses with the alpha motor neurons after entering the spinal cord, and travels back down the alpha motor neurons innervating the soleus muscle, where the H reflex CMAP is ultimately recorded (51). The H reflex can be considered as the electrical equivalent of the Achilles reflex; however, the muscle spindle is bypassed (52).
The Hmax/Mmax ratio is the ratio of the maximal H reflex to the maximal M response and has been investigated in several studies of patients with SCI (53–55). The Hmax/Mmax ratio is used to determine the percentage of motor neurons that are activated reflexively (H reflex) compared with those that are directly activated (M response); this gives an estimation of motor neuron excitability at rest. Some studies have shown an increased Hmax/Mmax ratio in individuals with SCI; however, there is little correlation between severity of spasticity and Hmax/Mmax ratios (52). Similar results can be obtained by substituting Tmax, the Achilles tendon jerk, for Hmax. The Tmax/Mmax ratio is similar to the Hmax/Mmax ratio, with the exception that rather than activating the Ia afferent fibers electrically as with the H reflex, the fibers are activated with a mechanically induced stretch to the muscle fibers when the Achilles tendon is tapped. Similar to the Hmax/Mmax ratio, the Tmax/Mmax ratio reflects motor neuron excitability, and there is not a correlation between it and the severity of spasticity (56); however, unlike the Hmax/Mmax ratio, the Tmax/Mmax ratio is influenced by the gamma system (6).
F waves are another late response that, similar to the H-reflex, reflect proximal conduction along the peripheral nerves. The F wave is elicited by supramaximal electrical stimulation of a mixed nerve while recording over a distal muscle innervated by that nerve. The electrical stimulus travels antidromically along the motor nerve, and once it reaches the axon hillock, a small percentage of the motor neurons backfire, and the action potential then travels orthodromically back down the motor neurons; this causes a late response that can be recorded over the distal muscle (51). Some studies have shown increased amplitude and persistence of F waves in individuals with spasticity, and this may reflect excitability of the motor neuron pool (52).
Spasticity may also be evaluated using electromyography (EMG). Sköld et al (57) evaluated LE spasticity in patients with SCI by using surface EMG to record muscle activity of the quadriceps and hamstrings during passive flexion and extension at the knee joint, as well as using the MAS to clinically assess the presence of spasticity. These researchers found a correlation between spasticity ratings on the MAS and electrical activity on surface EMG recordings; in addition, they noted that with each increasing grade on the MAS, there was evidence of increased myoelectric activity on EMG recordings.
The difficulties with quantitative tests to measure spasticity are attributed to a number of reasons. These include difficulty using a static test for a dynamic process; spasticity changes based on time of day and with many other factors (ie, stress, infections); test position usually is not the position of function for the patient; different scales measure different aspects of spasticity; individual tools correlate weakly with each other (5); discrepancy between self-rated and clinical scores; and a decrease in a score does not necessarily correlate with improved function. There is still no accepted measure that addresses the specific impact of spasticity in limiting activity or participation. Priebe has suggested that spasticity is best measured by a battery of tests that assess the different variables and the patient’s perspective (58).
TREATMENT
Spasticity is present in most individuals with SCI and may have a significant negative impact on QOL and functional activities (59). Little et al (9) found that 59% of patients with traumatic SCI reported that spasticity interfered with transfers, and 65% claimed that it disrupted their sleep. Lundqvist et al (60) noted that in patients with SCI, those with spasticity scored significantly worse on the Sickness Impact Profile for ambulation and feeding activities than those without. Other studies have not found a negative association between QOL and spasticity, and some have even reported a positive impact of spasticity. In a study by Sköld et al, 40% of individuals with spasticity reported that it had a positive impact (10), and another study found that 23% reported that the positive effects of spasticity outweighed the negative (44). Some individuals use their spasticity to assist with standing and positioning, as well as to aidthem in ADL, such as assisting with lower body dressing by eliciting flexor spasms in the LE. Fifty-three percent of individuals with tetraplegia and 26% with paraplegia report that they elicit spasms to assist with repositioning, dressing, transfers, and pressure relief (9). Some individuals with SCI find spasticity useful because it can serve as a warning sign that there is a change in their normal physiologic state, including the presence of a distended bladder, urinary tract infection, or pressure ulcer (61). In addition, spasticity may help to preserve muscle bulk by preventing atrophy (62). Overall, it seems that spasticity more often than not can have a negative impact on a patient’s life. Treatment should occur not because spasticity is present but rather because it interferes with specific activities and the treatment has the potential to address a passive or active functional goal. Because of the variable impact of spasticity on the QOL and functional status in individuals with SCI, it is important to determine the necessity of intervention, discuss treatment goals, and tailor treatment plans on an individual basis. Some of the most pertinent questions to ask include the following: Does the spasticity cause pain? Is it leading to contracture? Does it interfere with function or sleep? Does it affect QOL?
There are a variety of indications for treatment of spasticity, and the approach should be dictated by the patient’s overall functional status and symptom severity. Some of the aspects that should be considered include the severity of the spasticity, the scope (ie, whether the spasticity is focal, regional, or generalized), what the medical and cognitive status of the patient is, and taking into account the side effects of treatments as well as the cost–benefit ratio. Treatment goals vary from patient to patient and may range from maximizing gait in highly functional patients to goals of decreasing quantity of painful spasms or to improve the ease of care in patients who are dependent for their self-care needs (22).
Although it was previously taught that spasticity should be approached in a stepwise fashion, with initiation of noninvasive interventions including stretching and therapy programs before consideration of pharmacologic or surgical treatments, more recently the approach of using multiple interventions at the same time has been advocated; for example, utilizing modalities and medications at the same time. When increases in spasticity are noted in patients with a SCI that is stable, one must first consider potential causes of the change, such as presence of infection, bladder calculi, pressure ulcers, abdominal pathology, ingrown toenail, hemorrhoids, deep vein thrombosis, heterotopic ossification, or medication side effect, before initiating other interventions to treat the spasticity (6). Drug effects can serve as a source of newly increased spasticity, that is, selective serotonin reuptake inhibitors have been reported to increase spasticity (63). Changes in spasticity in an otherwise stable patient with a chronic SCI may also be an important presenting sign in syringomyelia (64). Spasticity-related interventions should be aimed at what matters most to the patient, improving comfort and function and allowing the individual to participate in life activities.
Nonpharmacologic Interventions
Initial treatment of spasticity typically involves nonpharmacologic, noninvasive measures. It is important to ensure that noxious stimuli do not contribute to the patient’s spasticity. Prevention of pressure ulcers and complications related to the patient’s neurogenic bowel and bladder by maintaining appropriate skin protocols and bowel and bladder management regimens will help to minimize noxious stimuli commonly encountered in this population. Positioning and stretching are also useful and necessary for decreasing spasticity and maintaining ROM at the affected joints and may even decrease long-term spasticity (6,65). The frequency, duration, and type of stretching that should be performed, as well as its long-term benefit, have been studied. A literature search on the effect of stretching showed that although there is some benefit to each stretching session, there is a wide diversity regarding its impact. Specifically, this review found that the available evidence on its clinical benefit is overall inconclusive (66). Tilt-table standing reduces spasticity in individuals with SCI, most likely by providing a prolonged stretch on the ankle plantar flexors (67), and has demonstrated efficacy in reduction of extensor spasms (68). Posture and adequate low back support are also important factors for tone reduction. Adequate low back support in the wheelchair to maintain lumbar lordosis and a positive seat plane angle or “dump,” with a reduction in seat-to-back angle, encourage proper upright posture and may reduce extensor tone.
Modalities
Other modalities and therapeutic interventions are available for the management of spasticity. Local cryotherapy has been reported to temporarily decrease spasticity, possibly due to temporary slowing of nerve conduction and reduction in the sensitivity of the muscle spindle fibers. The results obtained with local cooling typically diminish within 15 to 20 minutes after removal of the cold application (69). Electrical stimulation is another commonly used modality that is reported to decrease spasticity in individuals with SCI (70–72). Transcutaneous electrical nerve stimulation (TENS) is a technique that has been used in the management of chronic pain by applying the gate control theory (73), and has been shown to be beneficial for management of lower limb spasticity in individuals with SCI (74,75); its combination with physical therapy has also been found to be advantageous compared with 30 minutes of physical therapy alone (76). Several studies have described a reduction in spasticity of the lower limbs in individuals with stroke or SCI receiving TENS therapy over the spastic muscles or over corresponding spinal dermatomes (75,77–80). Functional electrical stimulation in addition to its other potential benefits has shown that it may be beneficial in reducing spasticity (81,82). In a crossover study comparing electrical stimulation to the LE in a small group of persons with SCI versus passive movement in an LE ergometer machine, spasticity as measured by the MAS and the pendulum test was significantly decreased (82). Electroejaculation using a rectal probe (83) as well as vibratory stimulation (84) have also shown to decrease spasticity in persons with SCI. Vibration applied locally over the rectus femoris resulted in localized improvement in ROM, MAS, and decreased duration of clonus (85). Transcutaneous spinal cord stimulation has been studied in a small group of patients (n = 3) and was found to have some spasticity control without negatively impacting motor control in incomplete injury (86). Whole body vibration has been shown to decrease quadriceps spasticity in chronic SCI when measured by the pendulum test (87), and increased cadence, step length, and walking speed by 0.063 m/s in chronic incomplete SCI (88); a recent systematic review has been published (89).
Orthotics
Tone-reducing orthotics are designed to improve gait patterns and decrease reflexive muscle activation in individuals with spasticity. There are several important design features of these orthotics that decrease spasticity in users, including the metatarsal pad and the great toe shelf that cause unloading of the metatarsal heads and extension of the great toe, delivery of constant pressure at the site of gastrocnemius and soleus insertion into the calcaneous, joint stabilization, and full contact with the muscle bellies provided by the thermoplastic material. The use of tone-reducing orthotics has previously been described in individuals with spasticity due to stroke and cerebral palsy, and a recent study demonstrated efficacy when used in individuals with SCI (90). Nash et al (90) demonstrated improvement in step length, gait velocity, and scores on the SCI-Functional Ambulation Inventory, as well as a reduction in abnormal EMG activity in the gastrocnemius in an individual with incomplete SCI and spasticity when using tone-reducing ankle foot orthoses bilaterally.
Nontraditional Interventions
Other alternative interventions may play a role in the reduction of spasticity in individuals with SCI. According to a small study, lower limb movement through passive cycling showed subjective LE spasticity improvement (91), electric passive cycling showed increased ROM and decreased mean MAS in the LE (92), and hippotherapy showed decreased AS and subjective reported improvement (93,94) in persons with SCI. The beneficial effects of hippotherapy on spasticity reduction are thought to be related to the saddle position, which places the patient’s LE into hip flexion, abduction, and external rotation; other benefits may be due to the effects of rhythmical trunk side flexion and extension produced during hippotherapy sessions. Massage has short-term reductions of spasticity (95). Body-weight-supported treadmill training has been shown to decrease flexor spasms (96). In another study, body-weight-supported locomotor training has been shown after 12 weeks to decrease ankle clonus and quadriceps spasms duration (97). Acupuncture has been studied as a potential intervention in SCI-related conditions, including motor deficits, pain, and spasticity. Electroacupuncture and stimulation of acupoints may be beneficial in the reduction of spasticity related to SCI, but further studies are needed (98). Hydrotherapy has also been studied in persons with SCI. In a randomized control study of 20 patients, exercise in a 71°F pool, three times per week for 20 minutes, demonstrated an increase in Functional Independence Measure score, a decrease in spasm severity, and a decrease in oral baclofen dose intake (99). Kinesio taping compared with non-elastic silk tape over the gastrocnemius and soleus muscles with 0% stretch has been shown in a small randomized crossover study to decrease MAS and clonus in individuals with chronic SCI (100).
Serial Casting
Serial casting involves a series of casts to reduce spasticity by stretching soft tissue and/or muscle lengthening. This can also be effective in stretching out a contracture. One can use the Tardieu Scale to know how much range one can achieve. The technique involves finding the end range, then slightly backing off to prevent tendonitis and improve tolerance to the cast. After 24 hours, the cast should be removed to check the skin. Subsequent casts can remain on for 2 to 3 days. The cast can be bivalved to allow for examination of the skin. Casting should continue until there is no improvement noted in two consecutive casts. To loosen the spasticity and improve the stretch, chemodenervation with localized injections can be administered first. Prolonged use of splints can be employed in place of the casts.
Pharmacologic Interventions
A variety of pharmacologic agents with different mechanisms of action are available for the treatment of spasticity of spinal cord origin; however, only four are Food and Drug Administration (FDA) approved for this use. These agents include baclofen, diazepam, dantrolene sodium, and tizanidine (101). A systematic review indicated that there is insufficient evidence to assist clinicians in a rational approach to antispasticity treatment in SCI (102), and therefore, some amount of trial and error is required. The person’s age, comorbidities, and cognitive status should be carefully considered when choosing a medication for spasticity. Centrally acting agents typically suppress excitation or enhance inhibition within the central nervous system, whereas peripherally acting agents act directly at the neuromuscular sites (Table 23.5).
Baclofen (Lioresal®). Baclofen is an antispasticity agent that is commonly used in the treatment of spasticity of spinal origin. Baclofen is considered by many to be the first line of treatment, although there are no studies definitively supporting this approach (102). Baclofen is a derivative of the inhibitory neurotransmitter GABA. It binds presynaptically to the GABA-B receptors in the brain and spinal cord and is thought to decrease monosynaptic and polysynaptic reflexes. Binding of baclofen to the GABA-B receptors decreases calcium influx into the presynaptic terminal; this in turn decreases the release of excitatory neurotransmitters. The decreased release of excitatory neurotransmitters by the interneurons and afferent fibers is responsible for the diminution of reflex activity (103). Binding of baclofen to GABA-B receptors may also decrease gamma motor neuron activity, which may decrease muscle spindle activity (101).
TABLE 23.5
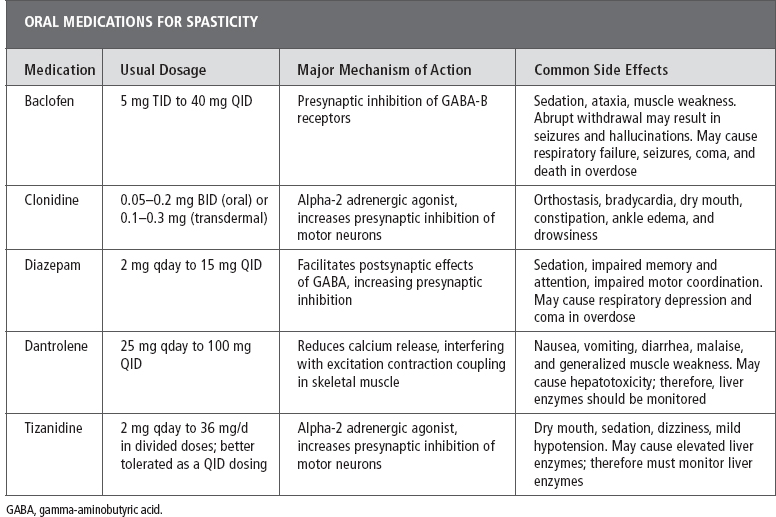
The mean half-life of baclofen is approximately 3 hours (range, 2–6 hours), necessitating dosing three to four times daily. Baclofen is excreted primarily by the kidney, although 15% is metabolized by the liver; therefore, caution must be exerted when using baclofen in patients with renal insufficiency, and liver function tests should be checked before initiation of treatment and periodically thereafter (101).
Baclofen can be administered orally and intrathecally (intrathecal dosing is discussed later). Oral baclofen should be initiated at a dose of 5 mg two to three times daily and gradually titrated up to an appropriate dose based on patient response and side effects. The package insert lists 80 mg as the maximum daily dose of baclofen; however, there are reports and clinical experience of using higher doses in SCI (104). A study by Aisen et al (105) showed that higher doses of baclofen (up to 240 mg/d) may be used safely in patients with spasticity due to MS or SCI. The authors of this chapter routinely titrate baclofen up to 80 mg/d in divided doses (as long as there is continued benefit) and feel comfortable increasing to higher doses (monitoring for side effects), often adding a second agent between 80 and 160 mg/d.
Several open-label studies have demonstrated the effectiveness of baclofen. Baclofen has been shown to decrease spasticity in 70% to 87% of patients with SCI or MS (101–106), as well as to decrease pain in both animal and human studies (103). Baclofen appears to have an anxiolytic property as well, as has been demonstrated in the SCI population (107) as well as individuals with panic disorder and chronic schizophrenia (108,109). Baclofen may also improve bladder function by decreasing outlet obstruction secondary to hyperreflexia of the external urethral sphincter (110). Baclofen has been compared to other antispasticity agents, and its effectiveness has been demonstrated to be equivalent to tizanidine in several studies. In studies comparing baclofen with diazepam, both were effective in decreasing spasticity; however, diazepam was more likely to cause sedation (106). As with many of the medications used for spasticity, although spasticity itself may be decreased, there is a paucity of literature documenting functional benefits. For this reason, dosages should be titrated based on improvement noted by patients in their daily routine.
Although baclofen is generally well tolerated, dose titration may be limited by side effects. Most commonly, baclofen can cause sedation and mental confusion, ataxia, hypotonia, and constipation. Due to the risk for cognitive side effects with the use of baclofen, one should exercise caution when using this medication in individuals with dual diagnosis of SCI with concomitant brain injury, and perhaps consider other alternatives. Respiratory failure, seizures, coma, and death have been reported after significant overdose. Abrupt withdrawal of baclofen should be avoided because it can result in seizures, hallucinations, and potentially death. Clinicians need to be especially mindful of this fact after the placement of an ITB pump. Because the amount of medication that is needed for spasticity management is so low, there is a real risk for baclofen withdrawal if the oral dose is weaned too quickly.
Concerns have been raised in recent years regarding the potential negative impact of baclofen on motor function. Angeli et al (111) report the case of an individual with incomplete tetraplegia, C5 AIS B classification, who converted to C5 AIS C after being weaned from baclofen. Clinical and EMG evaluations before the baclofen wean revealed no voluntary movement or EMG activity in the LE; however, on reevaluation post-wean and following 32 sessions of locomotor training, the patient had regained the ability to voluntarily move his toes and was also able to perform standing and squatting activities with body weight support assistance.
The effect of chronic use of baclofen on EMG activity and force within individual human thenar motor units was investigated by Thomas et al (112). These investigators evaluated force and EMG activity within single thenar motor axons in response to intraneural stimulation of the median nerve in individuals with chronic complete SCI at the C4, C5, or C6 level. Five subjects were assigned to the paralysis and baclofen group, as they had chronically used baclofen for spasticity control (median of 7 years), and the remaining seven subjects were assigned to the paralysis only group, as they were not taking baclofen. This study revealed that the thenar motor units in the individuals with paralysis and chronic baclofen were weaker compared with the group of paralyzed individuals who were not taking baclofen.
Recently Chu et al (113) investigated the effect of single doses of baclofen and tizanidine compared with placebo on motor reflexes in individuals with motor incomplete SCI. Reflexes and strength were measured before medication administration (30 mg baclofen, 4 mg tizanidine, and 10 mg placebo), and assessments were repeated 90 to 120 minutes after medication administration. The agents were tested in separate experimental sessions more than 1 week apart to allow adequate washout of the drug. These investigators found that administration of both tizanidine and baclofen resulted in reduction in stretch reflexes without reducing volitional torque. Given the limited number of studies investigating the effect of baclofen on motor strength and functional status, it is important for the clinician to weigh the risks and benefits when prescribing baclofen or other antispasiticity agents, and closely monitor the impact of these medications on patients’ motor and functional status.
Benzodiazepines. Diazepam (Valium®), a member of the benzodiazepine family, is one of the oldest antispasticity agents. Diazepam acts through the GABA system; however, its mechanism of action is different from baclofen in that it does not bind directly to the GABA receptor; rather, it is presumed to bind near the GABA-A receptor to indirectly facilitate the binding of GABA to the GABA-A receptors, thereby increasing presynaptic inhibition and reducing monosynaptic and polysynaptic reflexes.
Diazepam displays good oral absorption; after oral administration, blood levels peak within 1 hour. It is one of the long-acting benzodiazepines, with a half-life of 20 to 80 hours. Diazepam is metabolized in the liver, so it may cause side effects in individuals with liver dysfunction. In addition, diazepam is 98% protein-bound; so caution must be exercised when using this medication in individuals with low serum albumin (ie, acute SCI), as they may demonstrate increased susceptibility to side effects (101). Diazepam may be dosed starting at 2 to 5 mg when given at bedtime or 2 mg given during the daytime. Dosage should be gradually titrated up to a total daily dose of 40 to 60 mg (given in divided doses) as tolerated and required by the patient (6,101).
Diazepam has been shown to be effective in treatment of spasticity; however, it was noted to be more sedating than baclofen in a comparison study (106). Benzodiazepines may affect cognitive performance measures such as attention, concentration, and memory and are not recommended in persons with concomitant brain injury. Diazepam may be less effective in treating spasticity in individuals with complete SCI compared with those with incomplete SCI, as it appears that the brainstem reticular formation is more sensitive to the effects of diazepam than other spinal pathways (114–116).
Diazepam produces depression of the central nervous system, which can decrease the level of arousal, can cause sedation and impaired motor coordination, impair memory and attention, and may cause respiratory depression and coma in overdose. Diazepam may cause physiologic addiction, and abrupt discontinuation may result in a withdrawal syndrome, with symptom onset typically occurring 2 to 4 days after discontinuation. Typical withdrawal symptoms may include anxiety, agitation, nausea, and restlessness; however, severe cases can result in seizures and death (101). Because of the potential cognitive side effects associated with the use of diazepam, this medication should be avoided in those patients with SCI who have a concomitant brain injury.
Other benzodiazepine agents have been investigated for use in patients with spasticity. Ketazolam is a long-acting benzodiazepine given in a single daily dose of 30 to 60 mg. It has been studied in patients with spasticity secondary to MS, stroke, and brain injury and was noted to have equal efficacy and less sedating properties than diazepam (117,118). Clonazepam (Klonopin®) is another benzodiazepine agent that may be considered for use in the treatment of spasticity and is typically used for painful nocturnal spasms. The half-life is 18 to 28 hours, and it is initiated at doses of 0.25 to 1 mg at night and titrated up to a total dose of 3 mg as tolerated and required. In a study comparing baclofen and clonazepam in the treatment of spasticity related to MS, clonazepam demonstrated similar efficacy to baclofen; however, sedation, fatigue, and confusion were more common with clonazepam use (119).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






