Abstract
Rehabilitation aims to decrease neurological impairments, in guiding plasticity. Electrical stimulation has been used for many years in rehabilitation treatment of neurological disabilities as a tool for neuromodulation inducing plasticity, although the mechanisms of its action are not well known. The applications vary, encompassing therapeutic and rehabilitative aims. The type and site of stimulation vary depending on the objectives. Some techniques are widely used in clinical practice; others are still in the research stage. They may be invasive, epidural or in direct contact with neurons; they may be noninvasive, applied transcutaneously or indirectly by current vectors. The indications vary: mobility, functionality, pain as well as pharyngeal, respiratory, and perineal function. This paper aims to summarize current data on electrical neuromodulation techniques used in neurorehabilitation, their effects and their mechanisms of action.
1
Introduction
Stroke and spinal cord injury (SCI) are 2 of the principal causes of morbidity and mortality in adults. They are responsible for neurological impairments that can have an important impact on patient life and represent considerable costs for health and social services.
Rehabilitation aims to decrease neurological impairments and restore independence in activities of daily living. It has to be intense, repeated and task-oriented to facilitate motor recovery for hemiplegic or incomplete quadriplegic patients, in guiding plasticity . Indeed, brain plasticity is defined as all the mechanisms allowing the brain to adapt its own function to a new situation, such as a stroke. These mechanisms are a modification of the efficiency of existing synapses (long-term potentiation, long-term depression), global changes in post-synaptic excitability, and morphological changes (increase in number of dendrites or collateral axons). For example, changes in cortical maps have been widely reported after stroke . Functional recovery is based on the recruitment of areas adjacent to the lesion or remote areas of the lesion, which could be in the healthy hemisphere . Therefore, the opportunity to guide post-lesional plasticity is a key therapeutic goal for these patients . Neuromodulation techniques can induce brain plasticity by increasing or decreasing the excitability of a neuron or a brain area.
Electrical stimulation (ES) has been used for many years for neuromodulation in rehabilitation programs for patients with neurological impairments, although the mechanisms of action are not well known. The applications vary; the choice of parameters depends on the objectives. ES is defined by its polarity, width, intensity, frequency of stimulation, transcutaneous or invasive application, and stimulation site (the nerve, muscle), for example. It can be coupled with other tools such as electromyography (EMG), robotic devices, brain–computer interface techniques, or transcranial magnetic stimulation (TMS). Some techniques are used in clinical practice; others are still in preclinical development.
This paper aims to summarize current data on electrical neuromodulation techniques used in neurorehabilitation, their effects and their mechanisms of action. The results are presented according to the stimulation sites. The list is not exhaustive.
2
Brain ES
2.1
Invasive brain ES
2.1.1
Movement disorders
Invasive brain ES has been developed for more than 25 years, with many clinical applications, including pharmacological resistance, reducing disability and improving quality of life . Deep brain stimulation (DBS) involves intracerebral implantation of stimulation electrodes that are connected to a subcutaneous generator. The technique involves continuously delivering small electrical pulses to modulate the activity of the targeted brain area and related brain networks. The precise site of stimulation depends on the indication. The most frequent targets are the subthalamic nucleus, a key structure within the basal ganglia circuit in Parkinson’s disease , the ventral intermediate nucleus of the thalamus in severe tremor secondary to Parkinson’s disease or in essential tremor , and the globus pallidus internus in primary dystonia . For complex tremors, the DBS targets the ventral oralis posterior thalamus or the zona incerta. The clinical results vary .
2.1.2
Epilepsy
ES has been developed for drug-resistant epilepsy. Vagal nerve stimulation by microelectrodes may result in long-term seizure reduction. In cases of no benefit with vagal nerve stimulation, different sites of DBS have been proposed: the anterior nucleus and centromedian nucleus of the thalamus and mesiotemporal structures .
2.1.3
Psychiatric disorders
DBS is used clinically for depression by targeting the subgenual cingulate region, the ventral caudate nucleus and the ventral striatum and for obsessive–compulsive disorder by targeting the caudate nucleus, anterior limb of the internal capsule and nucleus accumbens .
2.1.4
Central neuropathic pain
Tsubokawa et al. introduced epidural motor cortex stimulation as an alternative treatment for the central neuropathic brain . Literature results are discordant, except for deafferentation facial pain, which responds positively to this ES .
2.1.5
Stroke
Epidural stimulation was investigated by Brown et al., who applied ES at 50 Hz through epidural electrodes implanted in relation to the cortical motor area of the wrist in 6 chronic hemiplegic patients for 3 weeks, coupled with rehabilitation . The results were promising, but tests have not been continued.
2.2
Noninvasive brain ES
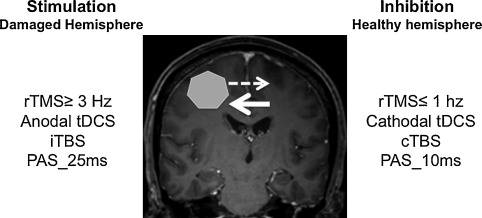
Noninvasive brain stimulation (NIBS) ( Fig. 1 ) has been developed for more than 10 years and includes transcranial direct current stimulation (tDCS), repetitive TMS (rTMS) and paired associative stimulation (PAS), which couples peripheral ES and cortical magnetic stimulation . These techniques are based on the concept of restoring interhemispheric balance after brain injury to modulate and promote functional recovery. Indeed, after stroke, functional imaging studies have shown hypoactivation of the ipsilesional cortical areas, followed over time by activation of ipsi- and contralesional sensorimotor areas and finally, a return to a more conventional circuit, so ipsilesional, which allows for better quality of recovery . The larger the interhemispheric asymmetry, the more limited the motor recovery. NIBS aims to stimulate the damaged cortex or inhibit the healthy cortex to restore interhemispheric balance.
2.2.1
tDCS
The technique consists of applying a galvanic, constant, continuous current at low intensity (1–2 mA) via 2 large non-metallic electrodes (5 × 7 cm) wetted by a saline solution. One of the electrodes is positioned in relation to the target area. Stimulation can involve the anode or cathode, called anodal or cathodal stimulation, respectively. The second electrode is usually positioned on the skin on the side of the contralateral fronto-orbital region . When applied for more than 3 min at 1 mA or 5 min at 0.6 mA, the stimulation induces excitatory after-effects if anodal or inhibitory after-effects if cathodal. Effects lasting beyond the duration of the stimulation define after-effects.
On the basis of the concept of restoring interhemispheric balance, to enhance motor recovery, tDCS is applied to the damaged primary motor area with anodal stimulation to stimulate it or with cathodal stimulation on the healthy side to inhibit it . Its impact on motor recovery was first assessed by Hummel et al., who showed that tDCS improved hand motor function as assessed by the Jebsen-Taylor Hand Function Test . In a recent Cochrane meta-analysis, Elsner et al. found some evidence of the effectiveness of tDCS for improving activities of daily living and function after stroke .
Similarly, the effects of tDCS have been studied in aphasia, with anodal stimulation applied to Broca’s area or cathodal stimulation of the right part of Wernicke’s area . Elsner et al. did not find any evidence of the effectiveness of tDCS for improving functional communication or language impairment .
More recently, some studies explored the effect of tDCS on spatial neglect by anodal stimulation of the injured posterior parietal cortex and in disorders of consciousness after head injury or cerebral anoxia by anodal stimulation of the left primary sensorimotor cortex or the left dorsolateral prefrontal cortex .
The benefit of tDCS for managing chronic neuropathic pain has not been demonstrated .
2.2.2
TMS
TMS is a safe, painless technique based on the application of magnetic waves to the scalp via a coil in single, paired or repeated pulse mode (single or paired pulse TMS, repetitive TMS [rTMS]) . The magnetic stimulator comprises a capacitor able to generate a high voltage (2–3 kV) and a very short (0.3–1 ms) electric current in a coil of copper wire . This variation in current in the coil creates a very short (1 s) but very intense (1.5–2 Tesla) magnetic field, with a peak at 100 μs. This magnetic stimulation induces an electric current in the brain. TMS is also a current vector. The technique notably allows for assessing the integrity of the corticospinal pathway. When stimulation is applied to the upper limb area of the motor cortex, the induced current preferentially activates interneurons, which in turn activate, via trans-synaptic ways, neurons in the corticospinal tract. The peripheral muscle response to this stimulation is registered by EMG as a motor-evoked potential.
Single-pulse TMS can be used to evaluate corticospinal tract conduction in clinical practice and motor cortical excitability: area of the motor-evoked potential, rest motor threshold, active motor threshold, cortical mapping, silent period, and intensity curve . Paired pulse TMS can be used to evaluate intracortical inhibitory or excitatory circuits (intracortical inhibition, intracortical facilitation) when applied to a single hemisphere and cortico–cortical connectivity (transcallosal inhibition) when applied to 2 hemispheres .
rTMS consists of applying a series of same-intensity magnetic stimulations for a given frequency, ranging from 1 to 50 stimuli/s, to a targeted cortical area. It modulates the cortical excitability by suppressing or facilitating the cortical process for a variable period after the end of stimulation (after-effects), depending on the stimulation parameters. It is used as a cortical neuromodulation tool inducing after-effects depending on the stimulation frequency. rTMS induces long-term and reversible changes in the excitability of the underlying cortex: low-frequency rTMS (≤ 1 Hz) decreases it and high-frequency rTMS (≥ 3 Hz) enhances it . A subtype of rTMS, theta burst stimulation (TBS), consists of delivering short bursts of 3 pulses at 50 Hz. Intermittent TBS, with TBS delivered in 2-s trains separated by 10 s, has excitatory effects, whereas continuous TBS causes inhibitory effects . The rTMS and TBS after-effects are considered to result from modulation of long-term depression and long-term potentiation, involving synaptic plasticity mechanisms .
Like tDCS, the use of rTMS is based on the concept of restoring the interhemispheric balance and it is applied to the motor cortical areas, the areas of language, the prefrontal cortex of the healthy hemisphere or the damaged hemisphere, according to the objectives . It can improve motor function in 10% to 20% of patients, especially those with subcortical stroke; the effects seem greater with low-frequency rTMS over the unaffected hemisphere than high-frequency rTMS over the damaged hemisphere . Concerning aphasia, low-frequency rTMS over the triangular part of the right inferior frontal gyrus (IFG) has a positive effect on language recovery . High-frequency rTMS over the damaged hemisphere seems also to have beneficial effects . Some studies have reported the benefit of rTMS for visuospatial neglect, targeting the prefrontal cortex .
2.2.3
PAS
PAS was first applied at the abductor policis brevis in healthy subjects . It consisted of the association of 2 stimuli: an electrical peripheral stimulus applied to the median nerve at an intensity of 300% of the perception threshold and a magnetic cortical stimulus (TMS) applied to the cortical motor area of the hand to induce lasting, reversible and specific brain plasticity. The time interval between the 2 stimuli (interstimuli interval [ISI]) is fundamental. If the ISI is about 25 ms, the effect is facilitation and if < 10 ms, inhibition. The action mechanisms are close to long-term potentiation and depression. In subsequent studies, according to the same principle, the ES was then applied as a pulse train to the motor point of the target muscle .
PAS has been proposed to facilitate upper extremity motor recovery after stroke (ES on the wrist extensor muscle ) or SCI (ES on the ulnar nerve ). Similarly, it was applied to lower limbs in patients with hemiplegia (ES on the peroneal nerve ) and incomplete SCI (ES on the tibial nerve ). The effects are primarily electrophysiological, inducing changes in cortical excitability. The technique is still at the preclinical stage.
In conclusion, more studies are necessary before NIBS is used in clinical practice to restore motor, cognitive or language functions. They will aim to define the factors that affect the efficacy of NIBS, the best NIBS, the hemisphere to target, the best time after the lesion to apply NIBS and the parameters of stimulation.
2.3
ES of the upper and lower limbs
Peripheral ES has been used for many years as a therapeutic intervention, inducing brain plasticity and improving function. Indeed, schematically, ES primarily stimulates cutaneous and proprioceptor receptors and/or activates muscle contraction and joint movements, which can increase the cortical excitability of the somatosensory and/or motor areas and induce long-lasting cortical plasticity . There are many modalities depending on the clinical purpose: transcutaneous electrical nerve stimulation (TENS) generally applied at a sensory or motor threshold and neuromuscular ES (NMES) and functional ES (FES) applied generally above the motor threshold.
2.3.1
Sensitive ES
The modulation of afferent information induces motor cortical plasticity, called sensorimotor transformation. Ridding et al. used peripheral nerve stimulation for 2 h in healthy subjects; motor cortical excitability was specifically increased . Somatosensory ES (SES) consists of applying a low (intensity below motor threshold), continuous electrical current with a long pulse width, which preferentially activates Ia fibres . Prolonged SES alone improved motor skills in patients with SCI or stroke and corticospinal excitability. In addition, the functional effects of motor training in rehabilitating tetraplegic patients were augmented .
Moreover, sensory TENS applied at high-frequency (100 Hz) to a nerve via surface electrodes and inducing only sensory stimulation has beneficial effects when combined with active motor exercises in hemiplegic patients . The effectiveness was highlighted in the gait training of such patients by reducing spasticity of plantar flexor muscles and increasing the strength of dorsiflexor muscles . Similar results have been reported in patients with incomplete SCI .
However, Veldman et al., in a recent review, concluded that SES increased corticospinal excitability up to 40% and motor performance up to 14%, with no correlation between these 2 features . The mechanisms of SES increasing motor function are unclear, and the optimal parameters (duration, intensity, frequency) are not well determined.
2.3.2
NMES
NMES refers to ES of an intact lower motor neuron to activate paralyzed or paretic muscles. It stimulates the nerve directly or the motor point of the nerve proximal to the neuromuscular junction . The strength of the resulting muscle contraction depends on 3 parameters: the amplitude and the pulse width determine the number of activated muscle fibres, and the minimal frequency to induce muscle response is > 12.5 Hz. Other parameters must be considered to prevent muscle fatigue and improve the comfort of patients: the ramp time (time for the stimulus to reach peak intensity and return to zero) and the duty cycle (intermittent stimulation, ratio of “on” time, and total cycle time).
However, there are some differences in the pattern of motor unit recruitment between voluntary contractions and contractions induced by NMES. In a voluntary movement, the motor units are recruited and de-recruited in a non-synchronous sequence, with initial recruitment of small units resisting fatigue; however, NMES preferentially recruits synchronously large-diameter nerve fibres that innervate preferential larger motor units and induce rapid muscle fatigue . This fatigue is enhanced by high stimulation frequencies.
NMES can be broadly divided into 2 categories: therapeutic ES (TES) and functional ES (FES).
2.3.2.1
Therapeutic ES (TES)
Therapeutic ES (TES) aims for improvement after the end of ES. Repeated ES of the muscle leading to repeated muscle contraction may also improve motor function and grip , for example, by its application to the extensor muscles of the wrist and fingers of hemiplegic patients and to the deltoid and bicep muscles of SCI patients .
2.3.2.2
Functional ES (FES)
Functional ES (FES) consists of applying ES to induce an action potential in the axon of peripheral nerves, generating muscle contraction to assist in the performance of functional activities during stimulation. Thus, FES activates the targeted muscle to improve functionality by supplementing a lost function. This technique has been used since the 1960s as a neural protheses for gait training of hemiplegic subjects, initially targeting the dorsiflexion of the ankle and then using multichannel stimulation . The technique has therapeutic effects (i.e., the effect continues after the end of stimulation) when used as a training modality . FES has a beneficial effect on the recovery of grasping and gripping after SCI or stroke in particular by associating it with EMG signals. However, the superiority over other physical therapy has not been shown . Similar results were reported for improving walking . The technique is still being developed; the stimulator can be external or implanted and it should not be too heavy. Associated orthotics have been developed to stabilize a joint during the muscular contraction to improve functionality . Muscle fatigue is one limitation. Newly developed multichannel stimulators reduce this phenomenon by asynchronously stimulating the fibres. However, the use of FES is limited by spasticity, atrophy and loss of motor units. The mechanisms of action are not well explained. The effectiveness of FES may be based on direct action on the lost function and changes in cortical excitability by inducing brain plasticity via afferent stimuli that modulate the motor cortex , synaptic action at the spinal level , or motor learning induced by repetition of movement.
2.3.3
ES coupled with other techniques
All these ES techniques can be coupled with others to optimize their efficiency, for example, to an EMG signal , a robotic device or a brain–computer interface (BCI) .
In EMG-triggered neuromuscular ES, the patient has to start the movement. The EMG signal of the muscular activity registered by the surface electrode triggers the ES. This therapy requires more personal motor control and cognitive abilities than simple ES of the muscle.
BCI control of a limb prosthesis is accomplished by acquiring neurophysiological signals associated with a motor process, analyzing these signals in real time, and translating them into commands for a limb prosthesis . The technique has been successfully applied to FES devices for the upper and lower limbs but is still being developed.
In conclusion, the use of ES allows for intensive rehabilitation based on the repetition of task-oriented movements. This use corresponds to the recommendations given in the literature for the rehabilitative management of stroke . However, the mechanisms of action and modalities of use are not yet established.
2.4
ES for swallowing disorders
Swallowing disorders are a frequent morbidity after stroke, with an incidence of 28% to 81% . They are then responsible for malnutrition, pneumonia and high risk of death . The weakness of the swallowing-related muscles is one of the most common abnormalities of swallowing physiology in dysphagic patients . NMES applied to swallowing muscles has been proposed to strengthen them and decrease dysphagia consequences. Freed et al. applied TENS to the neck muscles, inducing contraction of the swallowing muscles, improving the laryngeal rise and increasing muscle strength and thus swallowing . TENS has benefit in managing swallowing disorders in stroke patients . Moreover, purely sensory stimulation has been applied under the chin to increase afferents, modulate brain plasticity and improve swallowing function . Therefore, the ES has positive effects on the swallowing performance of stroke patients with dysphagia, especially with pure sensory stimulation (intensity adjusted at the sensory level) or by stimulating the infrahyoid muscles during swallowing .
Finally, Michou et al. developed a type of neuromodulation using PAS with peripheral ES applied to the pharynx and coupled with cortical magnetic stimulation on the motor area of the pharynx . The technique increased pharyngeal cortical excitability and improved swallowing function. These results are encouraging, but the improvement in swallowing is minor and more studies are needed to precisely define the type and modalities of ES in swallowing disorders.
2.5
ES for respiratory dysfunction
Respiratory complications are the main cause of morbidity and mortality in the acute phase of SCI, with an incidence of 36% to 83%. Patients with SCI above C4 are at high risk of dependency on mechanical ventilation . Moreover, for injuries below C4, respiratory dysfunction occurs despite a preserved phrenic motor system and diaphragm: paralysis of thoracic intercostal nerves reduces ventilatory efficiency , and paralyzed abdominal muscles can lead to impaired cough with associated atelectasis, infection, and compromised respiratory function . Respiratory dysfunction may be related to 3 factors: reduced vital capacity, retention secretions and autonomic dysfunction .
ES techniques were developed to supplement the loss of diaphragm function. Invasive direct ES of the phrenic nerve triggers diaphragmatic contractions, which has lower cost than conventional ventilation because of fewer episodes of infection and improves quality of life . Phrenic pacing must be proposed as soon as possible to limit the diaphragmatic amyotrophy, which is a factor of failure of ventilatory weaning . Direct diaphragmatic ES involves endoscopy used to implant electrodes directly within the diaphragmatic muscle and connected to an external stimulator implanted in the skin . However, these 2 techniques only supplement the inspiratory function and do not replace expiratory functions such as coughing or removal of secretions.
ES also can be used to strengthen pectoral and abdominal muscles to improve cough capacity .
Thus, ES has a place in the complex management of respiratory dysfunction.
2.6
Perineal ES
Perineal disorders are common in neurological diseases. They may be responsible for impaired quality of life but also serious complications such as severe sepsis or renal failure. Two neuromodulation techniques are possible: one invasive, which involves implanting an electrode into the posterior sacral roots, called posterior sacral neuromodulation, and another noninvasive, which consists of applying TENS on the tibial nerve.
2.6.1
Posterior sacral neuromodulation
This technique is indicated for urinary incontinence secondary to an overactive bladder and for urinary retention . Overall, 67% to 80% of patients achieve continence or greater than 50% improvement in urgent urinary incontinence symptoms with sacral nerve stimulation as compared with controls awaiting implantation . The electrode is implanted at the S3 root, from the third sacral foramen. The stimulation parameters are, in general, frequency 14 Hz, wavelength 210 μs and variable amplitude 0.5 to 20 mA. The management is in 2 stages: in a test period of 14 days, the electrode is connected to an external box; if the treatment is effective (usually defined by ≥ 50% clinical improvement), a permanent subcutaneous battery is then implanted. About one third of patients who undergo battery placement require surgical revision, most commonly for pain, lead migration, replacement or explantation of the pulse generator, or wound problems. The lifespan of the battery is approximately 5 years. This treatment in used in clinical practice, but the mechanisms of action are unclear. It is also indicated for anorectal disorders; the stimulation sites and stimulation parameters are the same .
2.6.2
TENS applied to the tibial nerve
This technique is indicated for overactive bladder . Two external electrodes are bonded in relation to the tibial nerve: one is located behind the medial malleolus and the second approximately 10 cm above. The current is bidirectional and the parameters are frequency 10 Hz, wavelength 200 μs and intensity about 20 mA. The objective is to modulate the sacral nerve plexus through the S2–S4 nerves. For multiple sclerosis, 3 months’ treatment achieved 80% improvement in clinical symptoms . However, few studies are available, and as for sacral neuromodulation, the mechanisms of action are unclear. Also, additional study is necessary.
2.6.3
Brindley’s sacral anterior-root ES
This technique, also called the sacral anterior-root stimulator implant, is indicated for complete SCI above D10 with a neurogenic bladder resistant to usual treatments . It is invasive and permanent and consists of implanting an electrode over the anterior sacral roots after laminectomy and posterior rhizotomy. The electrode is activated on request by an external housing, for urination. The posterior rhizotomy is necessary to avoid undesired reflexes during stimulation but is responsible for loss of erection and ejaculation reflex. The bladder stimulation can induce lower limb contractions that can be not well tolerated. Defecation is facilitated but not completely restored .
2.7
Spinal cord ES
To restore mobility in patients with SCI, 2 modalities of ES are being developed: epidural and intraspinal stimulation. With epidural stimulation, the electrodes are placed directly over the spinal cord. In 2 studies, prolonged epidural stimulation combined with intensive rehabilitation improved mobility of lower limbs . These results are promising but need to be confirmed with a larger number of patients. With intraspinal stimulation, the electrodes are implanted within the ventral grey matter of the spinal cord to directly activate alpha motor neurons . The first results in animals are encouraging .
2.8
Neurogenic pain and ES
ES has been used for many years to treat neuropathic pain. Direct stimulation of peripheral nerves results in decreased excitability, increased electrical threshold and transient slowing of conduction velocity . TENS is recommended for chronic pain; it exerts its effect via a local, spinal and supraspinal pathway . Low-frequency TENS exerts its effect via a descending inhibitory pathway and by modulating nociceptors (opioid, serotonin and cholinergic neurostransmitters), whereas high-frequency TENS activates the A beta fibres and increases the gate-control. In clinical practice, it is applied to the painful area or painful path. It can be used with 2 types of programs:
- •
the “gate-control” program involves the parameters impulse width < 100 μs, frequency 50 to 100 Hz, and intensity below the motor threshold. The effect is located, quick, but short;
- •
the endorphin program involves the parameters impulse width 200 to 500 μs, frequency 2 to 6 Hz, and intensity higher than the motor threshold. The effect is delayed but more prolonged than the gate-control program.
Spinal cord stimulation targeting the dorsal columns of the spinal cord is an alternative treatment for chronic neurogenic pain. Its efficacy and cost-efficiency are well known, but the mechanisms of action are unclear .
2
Brain ES
2.1
Invasive brain ES
2.1.1
Movement disorders
Invasive brain ES has been developed for more than 25 years, with many clinical applications, including pharmacological resistance, reducing disability and improving quality of life . Deep brain stimulation (DBS) involves intracerebral implantation of stimulation electrodes that are connected to a subcutaneous generator. The technique involves continuously delivering small electrical pulses to modulate the activity of the targeted brain area and related brain networks. The precise site of stimulation depends on the indication. The most frequent targets are the subthalamic nucleus, a key structure within the basal ganglia circuit in Parkinson’s disease , the ventral intermediate nucleus of the thalamus in severe tremor secondary to Parkinson’s disease or in essential tremor , and the globus pallidus internus in primary dystonia . For complex tremors, the DBS targets the ventral oralis posterior thalamus or the zona incerta. The clinical results vary .
2.1.2
Epilepsy
ES has been developed for drug-resistant epilepsy. Vagal nerve stimulation by microelectrodes may result in long-term seizure reduction. In cases of no benefit with vagal nerve stimulation, different sites of DBS have been proposed: the anterior nucleus and centromedian nucleus of the thalamus and mesiotemporal structures .
2.1.3
Psychiatric disorders
DBS is used clinically for depression by targeting the subgenual cingulate region, the ventral caudate nucleus and the ventral striatum and for obsessive–compulsive disorder by targeting the caudate nucleus, anterior limb of the internal capsule and nucleus accumbens .
2.1.4
Central neuropathic pain
Tsubokawa et al. introduced epidural motor cortex stimulation as an alternative treatment for the central neuropathic brain . Literature results are discordant, except for deafferentation facial pain, which responds positively to this ES .
2.1.5
Stroke
Epidural stimulation was investigated by Brown et al., who applied ES at 50 Hz through epidural electrodes implanted in relation to the cortical motor area of the wrist in 6 chronic hemiplegic patients for 3 weeks, coupled with rehabilitation . The results were promising, but tests have not been continued.
2.2
Noninvasive brain ES
Noninvasive brain stimulation (NIBS) ( Fig. 1 ) has been developed for more than 10 years and includes transcranial direct current stimulation (tDCS), repetitive TMS (rTMS) and paired associative stimulation (PAS), which couples peripheral ES and cortical magnetic stimulation . These techniques are based on the concept of restoring interhemispheric balance after brain injury to modulate and promote functional recovery. Indeed, after stroke, functional imaging studies have shown hypoactivation of the ipsilesional cortical areas, followed over time by activation of ipsi- and contralesional sensorimotor areas and finally, a return to a more conventional circuit, so ipsilesional, which allows for better quality of recovery . The larger the interhemispheric asymmetry, the more limited the motor recovery. NIBS aims to stimulate the damaged cortex or inhibit the healthy cortex to restore interhemispheric balance.











