and with no other cause of hypotension characterizes septic shock. For adults, a systolic arterial pressure of <90 mm Hg or a drop of >40 mm Hg from baseline defines hypotension. For children, septic shock includes tachycardia, decreased peripheral pulses compared to central pulses, and diminished urine output.
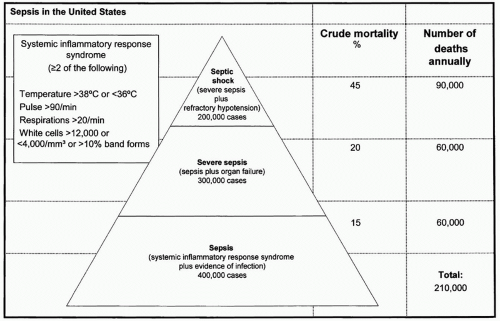 Figure 1 Sepsis in the United States. (Adapted from Manns BJ, Lee H, Doig CJ, et al. An Economic Evaluation of Activated Protein C Treatment for Severe Sepsis. N Engl J Med. 2002; 347(13):993-1000.) |
sinusitis. For 20% to 30% of patients, the site of infection may not be identified.3 In patients undergoing more specialized procedures such as maxillofacial reconstruction, spine fixation or craniotomies, closed space infections of the sinuses, or intracranial space should be considered if other, more common sources are not identified as the infectious source.
TABLE 1 SIGNS AND SYMPTOMS SUGGESTIVE OF SEPSIS | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
lavage (BAL) and quantitative cultures to decrease the chance of treating colonization.21,22,23 Use of “mini-BAL,” where a catheter is passed deep into the airway and blindly lavaged, has been used by some, but comparison to regular BAL is recommended to ensure reliability in one’s own practice. Also, in patients with infiltrates on one side of the chest radiograph, a mini-BAL may not accurately diagnose the infectious process because it may not sample the affected side.
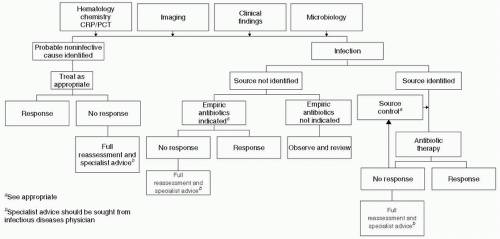 Figure 2 Algorithm for evaluation of suspected sepsis. (Adapted from Llewelyn M, Cohen J. Diagnosis of infection in sepsis. Intensive Care Med. 2001;27:S10-S32.) |
increased mortality.25 In addition, inappropriate antibiotic therapy has also been shown to increase morbidity and mortality from survival rates of 63% to 92% for correct antibiotic therapy down to 10% to 50% for ineffective antibiotic regimens.26,27,28 When selecting empiric antibiotic therapy, clinicians should take into account the following factors: likely site of infection, likely organisms, history of recent antibiotic use (which may increase resistance), culture data if known, drug penetration into the suspected site of infection, dose, and frequency of dosing.28,29 Because the speed of delivery and the appropriateness of antibiotics affect patient outcomes, broad-spectrum coverage of likely pathogens should be empirically started. When culture and sensitivity data become available, the antibiotic regimen can then be adjusted. Lack of correct initial antibiotic coverage for offending pathogens is a primary risk for increased mortality. Even with the correct antibiotics in the face of septic shock, delays in antibiotic administration increase mortality. In 2,154 septic shock patients who received correct antibiotic therapy after onset of hypotension, each hour of delay was associated with a decrease in survival of 7.6%. Septic shock patients receiving antibiotics in the first hour after onset of hypotension had a survival rate of 79.9%, but only 50% of patients received effective antimicrobial therapy within the first 6 hours.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







