Single-Bundle Anterior Cruciate Ligament
James S. Starman
Austin J. Crow
Mark D. Miller
DEFINITION
Anterior cruciate ligament (ACL) injuries result in a disruption of the fibers of this ligament and an ACL-deficient knee.
Although most injuries are complete, partial injuries have been described. In our practice, partial injuries—defined as an asymmetric Lachman test (or 3 to 4 mm of asymmetry on KT-1000 testing)1 with a negative pivot shift test during examination under anesthesia or a one-bundle ACL disruption seen arthroscopically—are rare.
The key point in determining how to treat partial injuries is to determine whether functional stability of the ACL has been maintained.
ANATOMY
The ACL is about 33 mm long and 11 mm in diameter.10
The tibial insertion has a broad, irregular diamond shape and is immediately anterior and adjacent to the medial tibial eminence.
The femoral attachment of the ligament is a semicircular area on the posteromedial aspect of the lateral femoral condyle.
It extends from the 9 o’clock position to the 11 o’clock position with the knee at 90 degrees flexion (right knee).
The ACL is composed of two “bundles”—the anteromedial (AM) portion, which is tight in flexion, and the posterolateral (PL) portion, which is tight in extension. The AM bundle functions more to control stability in the anteroposterior (AP) direction, whereas the PL bundle contributes significantly to rotational stability. It is composed of 90% type I collagen; the remaining collagen is predominantly type III.
The main blood supply for the ACL is the middle geniculate artery.
Mechanoreceptor nerve endings have been identified within the ACL and are thought to have a proprioceptive role.
PATHOGENESIS
ACL injuries occur frequently in sports that involve running, jumping, and cutting movements. They can occur without contact when the foot is anchored to the playing surface—usually by way of cleats or a rubber sole—and the body rotates beyond the tolerance of the ligament as the knee buckles. They also occur commonly when landing after a jump (untrained female athletes may land in valgus and extension).
Combined ACL, medial collateral ligament (MCL), and meniscal injuries have been referred to as the unhappy triad.17 Although the original triad included medial meniscal injuries, which are more common in chronic ACL injuries, lateral meniscal injuries are actually more common in acute ACL injuries (especially in skiers).4
NATURAL HISTORY
Researchers from Kaiser Permanente in Southern California, including Donald Fithian8 and the late Dale Daniel,7 have done much to contribute to our knowledge of the natural history of the ACL-injured knee. From their work, we recognize that patients with a high level of participation in jumping or cutting sports and significant side-to-side differences (>5 mm) on KT-1000 arthrometer measurements are at high risk for recurrent injury without ACL reconstruction.
Unfortunately, these same researchers have shown an increased incidence of arthritis in the surgically reconstructed ACL group.7,8
The difficulty with these and other studies is that multiple variables are involved, making comparisons difficult and possibly inaccurate.16
It is clear from the literature that the incidence of meniscal tears and chondral injury can be reduced with ACL reconstruction.
Advocates of double-bundle ACL reconstruction propose that the incidence of arthritis may be reduced with this technique but that theory has yet to be proven clinically.
PATIENT HISTORY AND PHYSICAL FINDINGS
Patient history may include the following:
Patients may describe a noncontact pivoting injury, typically involving a change of direction or deceleration maneuver.
Patients often recall hearing or feeling a “pop” and will develop an acute or subacute effusion (ie, “swells up like a balloon”).
In most cases, the athlete will not be able to return to play and may need assistance to leave the field or slope (we have termed the latter a positive ski patrol sign).
Physical examination methods include the following:
Effusion: About 70% of acute hemarthrosis cases represent ACL tears.7
Range of motion (ROM): Loss of extension may be a result of a displaced bucket-handle meniscal tear or arthrofibrosis (stiff knee). Loss of flexion may be related to a knee effusion.
The Lachman test20 is highly sensitive for ACL deficiency. The patient must relax for this examination, and effusion or a displaced meniscal tear may give a false end point.
The anterior drawer test is poorly sensitive and outdated but is helpful to rule out a posterior cruciate ligament (PCL) injury.
The pivot shift test3 is difficult to perform in the clinic setting but is an especially helpful and sensitive test during examination under anesthesia.
A complete examination of the knee also should include evaluation of associated injuries and ruling out differential diagnoses, including (but not limited to) the following:
Meniscal tears: Joint line tenderness, pain or popping with provocative maneuvers (eg, McMurray, Apley compression, duck walk), and loss of full extension may be present.
PCL injury: A “pseudo-Lachman” may be appreciated if the PCL is present, and the unwary examiner may falsely attribute this to an ACL injury. The key is the starting point on the drawer examination. The tibial step-off in PCL-injured knees will be absent, or the tibia may actually be displaced (or be displaceable) posteriorly, signifying a PCL injury.
Posterolateral corner (PLC) injury: Injury to the popliteus, popliteofibular ligament, biceps, iliotibial band, or posterior capsule will result in external rotation asymmetry (dial test), a positive PL drawer test, and external rotation recurvatum.
Collateral ligament injury: MCL injuries are recognized as opening with valgus force and lateral collateral ligament (LCL) injuries open with varus stress. These examinations are tested in both 0 and 30 degrees of knee flexion. Opening to valgus or varus stress in 0 degree (ie, full extension) signifies a more severe injury, usually involving one or both cruciate ligaments.
Patellar instability: Localized tenderness or instability with apprehension testing is essential to rule out a patellar dislocation that reduced spontaneously. This type of injury also can cause an acute knee effusion and can be easily confused with an acute ACL injury.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Plain radiographs, including AP, lateral, and patellar views, should be obtained to rule out bony avulsion fractures or associated injuries.
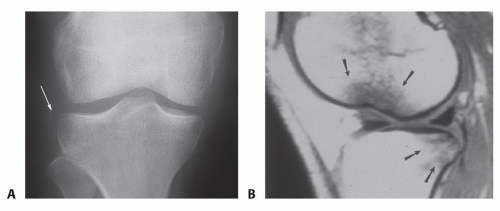
A small avulsion fracture off the lateral tibial plateau (FIG 1A) represents a lateral capsular avulsion (Segond sign) and is highly associated with an ACL injury. It is very specific but not sensitive.
Flexion weight-bearing radiographs are important in older or posttraumatic patients to rule out associated osteoarthritis.
Long-leg, hip-to-ankle radiographs must be obtained in patients with varus or valgus malalignment.
An osteotomy should be performed before ACL reconstruction in select cases.
Magnetic resonance imaging (MRI) is highly sensitive and specific in diagnosing ACL tears as well as associated injuries.
Bone contusions, or bruises, also may be detected in the midlateral potion of the lateral femoral condyle (near the sulcus terminalis) and the posterior tibial plateau (FIG 1B).
DIFFERENTIAL DIAGNOSIS
Meniscal tear
Osteochondral injury
Contusion
Patellar dislocation
Other ligament/capsular injury (eg, MCL, LCL, PLC, multiple ligament injury)
NONOPERATIVE MANAGEMENT
Although nonoperative management is controversial, patients with less laxity and those who are less involved with high-level pivoting sports may be treated nonoperatively.9
Nonoperative treatment is done in three phases over a period of about 3 months.
In the initial phase, emphasis is placed on regaining full motion, controlling effusion, and maintaining quadriceps tone. (This is appropriate for patients who are surgical candidates as well.)
In the second phase, quadriceps and hamstring strengthening is emphasized.
In the third and final phase, sport-specific rehabilitation is accomplished.
Patients may attempt to return to sports after their effusion has completely resolved, they have full ROM, their quadriceps tone and strength have been restored (isokinetic testing is helpful), and they have no residual symptoms of instability (functional testing is helpful).
SURGICAL MANAGEMENT
Preoperative Planning
All imaging studies are reviewed.
Plain radiographs should be reviewed for fractures, loose bodies, patellar height and alignment, and the presence of any hardware (from previous procedures) or foreign bodies.
Associated fractures, meniscal tears, articular cartilage lesions, and multiple ligament injuries should be addressed concurrently.
Examination under anesthesia should be accomplished prior to positioning.
Lachman, pivot shift, varus/valgus, and dial testing should be included in the examination under anesthesia.
Positioning
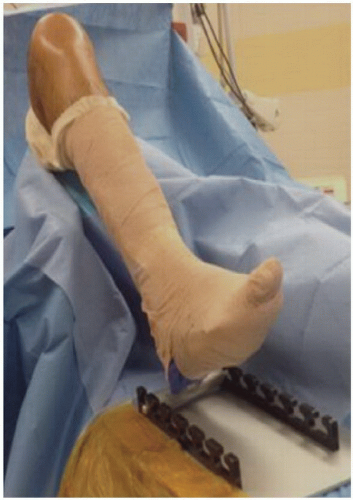
Although some surgeons prefer to perform ACL reconstruction with the patient’s operative knee in a knee holder with the foot of the bed dropped, we prefer to keep the patient supine with the foot of the table up and a post on the lateral side (FIG 2).
In patients electing for a hamstring autograft reconstruction technique, we routinely prep both lower extremities for potential bilateral graft harvest.
Table 1 Anterior Cruciate Ligament Graft Choice Indications | ||||
|---|---|---|---|---|
|
Approach
The approach depends on graft choice.
There are two gold standards for ACL grafts—bone-patellar tendon-bone autograft and four-strand semitendinosus gracilis (hamstring) autograft. We use both patellar tendon and hamstring grafts and have found that certain parameters are helpful in determining graft choice (Table 1).
Other graft choices include quadriceps tendon autograft and a variety of allografts. Although these grafts may be useful in certain cases, they are not popular choices for most surgeons. Recent studies have suggested a slightly higher failure rate with the use of allograft, particularly in younger and more active patients.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree