Shoulder Arthroplasty in the Young, Active Patient
Edward W. Lee
Evan L. Flatow
Jon J. P. Warner
E. W. Lee: Clinical Shoulder Fellow, Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
E. L. Flatow: Lasker Professor of Orthopaedic Surgery, Chief of Shoulder Surgery, Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
J. J. P. Warner: Professor of Orthopaedic Surgery, Chief, The Harvard Shoulder Service, Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts.
INTRODUCTION
As success with shoulder arthroplasty has grown, and orthopedists have become more familiar and adept with the technique, the indications for shoulder arthroplasty have expanded. Those patients refractory to an extensive trial of nonoperative treatment and unsuitable for, or having failed, arthroscopic treatment may be considered for prosthetic replacement. In young patients with severe proximal humerus fractures; avascular necrosis (AVN); and posttraumatic, postsurgical, inflammatory, and degenerative arthritides, humeral head replacement (HHR) and total shoulder arthroplasty (TSA) remain viable options.20,31,32,34 It is critical, however, to remember the limits of the technique; to remain clear on the indications and contraindications; and to accurately educate patients on the risks, benefits, alternatives, and expected outcomes of shoulder arthroplasty. This is especially the case in a young, active patient who may have expectations for active participation in sports and work for many years.
CAUSE
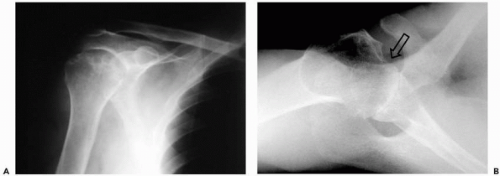
Cause plays an important part in the natural history and prognosis after arthroplasty. The shoulder joint is an uncommon site of primary osteoarthritis (OA). Osteoarthritis in the shoulder presents with large inferior osteophytes, sclerosis, subchondral cysts, and posterior erosion of the glenoid (Fig. 23-1). In young patients, glenohumeral OA is less common than arthritis secondary to trauma38 or prior instability repair. Humeral trauma or surgery that alters the biomechanics and results in humeral roughness or eccentric loading of the glenoid can eventually progress to glenoid arthrosis.31,34,40 Furthermore, associated soft-tissue insufficiency or contractures may be relevant to the overall pathology that will require treatment. Roentgenographic findings do not necessarily correlate with symptoms.
In rheumatoid arthritis (RA), the glenohumeral joint is commonly involved and usually part of a polyarthropathy3 (Fig. 23-2). Symptomatic RA of the shoulder typically occurs in women between 35 and 55 years-old who test positive for rheumatoid factor, with rotator cuff tears occurring in approximately 25% of these patients.38
Avascular necrosis can occur in patients with trauma, steroid use, alcoholism, sickle cell disease, hemophilia, history of decompression sickness, lipid storage diseases, or lupus.18,24,34 Often the cause is unknown. Disease progression was staged by Cruess as follows: preradiographic stage (I), humeral head involvement (II), subchondral humeral head fracture (crescent sign, III), humeral head collapse
(IV), and glenoid involvement (V) (Fig. 23-3). The later stages of AVN are typically more symptomatic, are more likely to progress, and most often require prosthetic replacement.24
(IV), and glenoid involvement (V) (Fig. 23-3). The later stages of AVN are typically more symptomatic, are more likely to progress, and most often require prosthetic replacement.24
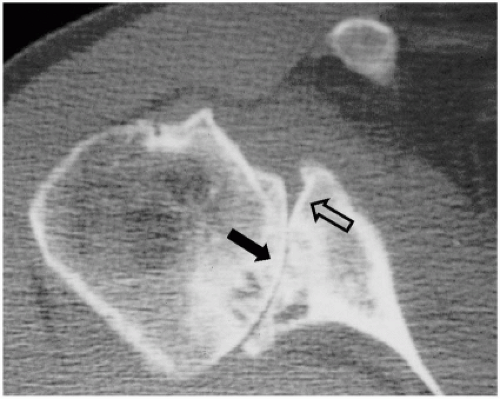 Figure 23-1 Computed tomography scan showing eccentric posterior glenoid wear in patient with osteoarthritis (OA). Native glenoid contour (hollow arrow). Posterior eroded contour (solid arrow). |
Degenerative glenohumeral arthritis can result from glenohumeral instability and is the most common cause of arthritis in patients less than 40 years-old.6,11 This may result from postsurgical, iatrogenic subluxations; persistent multidirectional instability; inadequate or inappropriate surgery; or a hardware complication. The cause is often multifactorial in nature11 (Fig. 23-4). In a series by Bigliani et al.,6 symptomatic arthrosis required prosthetic replacement at an average of 16 years after the initial stabilization.
EVOLUTION OF GLENOHUMERAL PROSTHETIC REPLACEMENT FOR ARTHRITIS
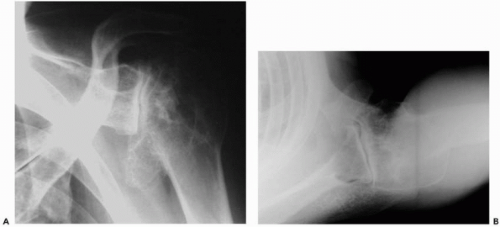
A number of changes have occurred in prosthetic design and technology as well as surgical technique in shoulder arthroplasty. First-generation arthroplasty for the shoulder used a “monoblock” design with few options to accommodate the variable anatomy between different patients (Fig. 23-5). Despite this lack of prosthetic variability, the “old design” performed well with Torchia et al.42 providing relief of moderate to severe pain in 83% of patients’ shoulders in their study.
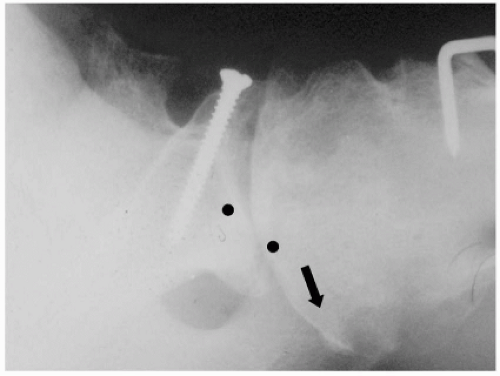
Second-generation shoulder arthroplasty introduced humeral head modularity and more stem sizes, which theoretically allowed for better soft-tissue tensioning. A number of various pegged and keeled glenoid designs were also introduced in an attempt to optimize fixation of the polyethylene component. Several problems with second-generation components became apparent, including nonanatomic head sizing, which prevented the restoration of proper offset and resulted in suboptimal cuff tension, overstuffing the joint, and eccentric glenoid loading. Survival problems became evident with early glenoid osteolysis and may have been multifactorial (overstuffing the joint, head/glenoid curvature mismatch, and material failure [i.e., Hylamer, DePuy, Warsaw, IN]) (Fig. 23-6).43 Humeral-sided complications arose as well, with stressshielding and proximal bone loss from large and stiff stems.
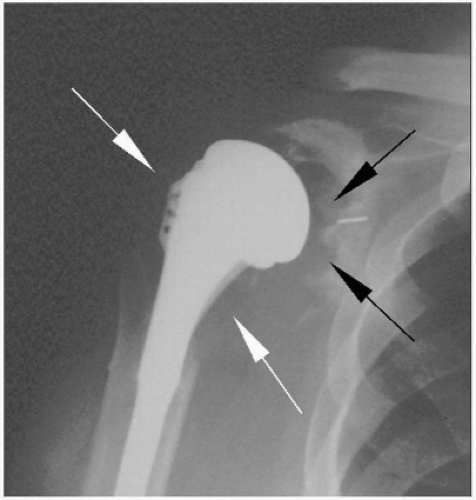 Figure 23-6 Second-generation shoulder prosthesis. Note severe osteolysis of the proximal humerus (white arrows) and glenoid (black arrows). |
Third-generation, or “anatomic,” systems have more prosthetic adaptability with offset heads and variable neck angles, better glenoid surface designs, and newer instrumentation to minimize bone loss and improve cement integration (Fig. 23-7, A and B). Better restoration of anatomy may minimize or avoid eccentric loading of the glenoid component or reduce glenoid wear after an HHR (Fig. 23-8, A and B).
HUMERAL HEAD REPLACEMENT VERSUS TOTAL SHOULDER ARTHROPLASTY WITH CONSIDERATIONS IN THE YOUNG, ACTIVE PATIENT
Currently, some confusion still exists in defining the indications for resurfacing the glenoid in glenohumeral arthritis. Advantages of hemiarthroplasty include a relatively easier procedure, a shorter operating time with less blood loss, less risk of instability, and the ability to convert to a TSA in the future. However, the disadvantages include less consistent pain relief and progressive erosion of the glenoid leading to deteriorating functional results over time (Figs. 23-9 and 23-10).
In contrast, TSA provides more consistent pain relief and restores the glenohumeral fulcrum, providing better active motion. Arguments against resurfacing the glenoid include a more difficult procedure with longer operative time and greater blood loss and glenoid wear resulting in bone loss and loosening of both components. The surgical technique has been well described in the literature.26
A number of current studies examining the clinical results of HHR versus TSA have favored glenoid resurfacing. In their short-term follow-up of 600 TSAs and 89 HHRs for primary OA, Edwards et al.19 found superior results with
TSA compared with HHR with statistically significant differences with respect to pain, mobility, and activity. Similarly, Bell and Gschwend5 found better average elevation and pain relief with glenoid resurfacing in their study of 11 TSAs and 17 HHRs. In their randomized clinical series, Gartsman et al.,21 Sandow et al.,37 and Kirkley et al.27 found better pain relief with TSA but no significant differences in functional outcome with HHR at short-term follow-up.
TSA compared with HHR with statistically significant differences with respect to pain, mobility, and activity. Similarly, Bell and Gschwend5 found better average elevation and pain relief with glenoid resurfacing in their study of 11 TSAs and 17 HHRs. In their randomized clinical series, Gartsman et al.,21 Sandow et al.,37 and Kirkley et al.27 found better pain relief with TSA but no significant differences in functional outcome with HHR at short-term follow-up.
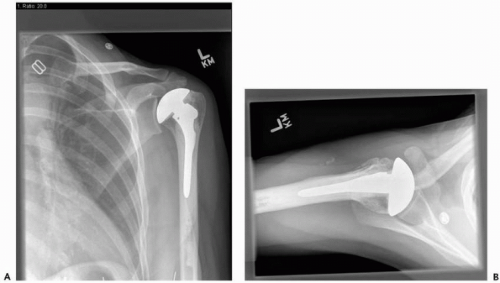 Figure 23-8 View of 35-year-old woman 3 years after hemiarthroplasty for avascular necrosis (AVN) with anatomic restoration of articular humeral anatomy. There is no joint space narrowing in anteroposterior (AP) view (A) or axillary view (B), and the patient has motion symmetric with contralateral normal side.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|