There has been remarkable growth in the development and application of robotics to ameliorate or remediate impairment. This growth is associated with a) the understanding that plasticity is a fundamental property of the adult human brain and might be harnessed to remap or create new neural pathways and b) that robots that can safely interact with humans and assist human performance. This article discusses whether robotic therapy has achieved a level of maturity to justify its broad adoption as a rehabilitative tool. How to improve outcomes further and how to select degrees of freedom to optimize care to particular patients is also discussed.
Key points
- •
Robot-assisted therapy for the upper extremity has already achieved class I, level of evidence A for stroke care in the outpatient setting and care in chronic care settings.
- •
At least in the US Department of Veterans Affairs (VA) health care system, robot-assisted therapy for the upper extremity has not increased the total health care utilization cost.
- •
Functionally based robotic training did not demonstrate any advantage over impairment-based robotic training.
- •
The paradox of diminishing number of degrees of freedom (DOFs) suggests an approach to tailor therapy to a particular patient’s needs.
Introduction: disruptive technology
Three years ago, the authors discussed the concept of disruptive technology and rehabilitation robotics (parts of this review have been published elsewhere). As described then and replicated in this article, disruptive technology is a term coined to characterize an innovation that disrupts an existing market or way of doing things and creates a new value network. The concept was introduced by Christensen and colleagues, who described the concept in 1996 as “Generally, disruptive innovations were technologically straightforward, consisting of off-the-shelf components put together in a product architecture that was often simpler than prior approaches. They offered less of what customers in established markets wanted and so could rarely be initially employed there. They offered a different package of attributes valued only in emerging markets remote from, and unimportant to, the mainstream.” Eventually with improvement, borrowing from Malcolm Gladwell, the moment of critical mass (the threshold or the boiling point) is reached and the old practices and existing value network abandoned in favor of the new one, also referred to “the tipping point.”
Introduction: disruptive technology
Three years ago, the authors discussed the concept of disruptive technology and rehabilitation robotics (parts of this review have been published elsewhere). As described then and replicated in this article, disruptive technology is a term coined to characterize an innovation that disrupts an existing market or way of doing things and creates a new value network. The concept was introduced by Christensen and colleagues, who described the concept in 1996 as “Generally, disruptive innovations were technologically straightforward, consisting of off-the-shelf components put together in a product architecture that was often simpler than prior approaches. They offered less of what customers in established markets wanted and so could rarely be initially employed there. They offered a different package of attributes valued only in emerging markets remote from, and unimportant to, the mainstream.” Eventually with improvement, borrowing from Malcolm Gladwell, the moment of critical mass (the threshold or the boiling point) is reached and the old practices and existing value network abandoned in favor of the new one, also referred to “the tipping point.”
Upper extremity robotic therapy: the tipping point
Since the publication of the first controlled study with stroke inpatients, several studies have been completed with both stroke inpatients and outpatients demonstrating the potential of robotic therapy for the upper extremity. These results were discussed in different meta-analyses (for example, in Refs. ) and led to the 2010 American Heart Association (AHA) guidelines for stroke care: “Robot-assisted therapy offers the amount of motor practice needed to relearn motor skills with less therapist assistance… Most trials of robot-assisted motor rehabilitation concern the upper extremity (UE), with robotics for the lower extremity (LE) still in its infancy… Robot-assisted UE therapy, however, can improve motor function during the inpatient period after stroke.” AHA suggested that robot-assisted therapy for the upper extremity has already achieved class I, level of evidence A for stroke care in the outpatient setting and care in chronic care settings. It suggested that robot-assisted therapy for upper extremity has achieved class IIa, level of evidence A for stroke care in the inpatient setting. Class I is defined as “Benefit >>> Risk. Procedure/Treatment SHOULD be performed/administered” (where >>> indicates that “much larger than”); class IIa is defined as “Benefit >> Risk, IT IS REASONABLE to perform procedure/administer treatment” (where >> indicates “larger than”); and level A is defined as “Multiple populations evaluated: Data derived from multiple randomized controlled trials (RCTs) or meta-analysis.”
The 2010 VA/Department of Defense (DOD) guidelines for stroke care came to the same conclusion endorsing the use of rehabilitation robots for the upper extremity but went further to recommend against the use of robotics for the lower extremity. More specifically, the VA/DOD 2010 guidelines for stroke care “Recommend robot-assisted movement therapy as an adjunct to conventional therapy in patients with deficits in arm function to improve motor skill at the joints trained.” More needs to be done, however, particularly for the lower extremity, as stated in the VA/DOD guidelines: “There is no sufficient evidence supporting use of robotic devices during gait training in patients post stroke” and “Recommendation is made against routinely providing the intervention to asymptomatic patients. At least fair evidence was found that the intervention is ineffective or that harms outweigh benefits.”
Currently the largest single study of upper extremity robotics confirms these endorsements for the upper extremity. The multisite, independently run, VA trial CSP-558 (Robotic Assisted Upper-Limb Neurorehabilitation in Stroke Patients [VA ROBOTICS]), on upper extremity rehabilitation robotics, using the commercial version of the MIT-Manus robot for shoulder and elbow therapy together with corresponding antigravity, wrist, and hand robots, included 127 veterans with chronic stroke at least 6 months post–index stroke with an impairment level characterized by very severe to moderate (Fugl-Meyer assessment between 7 and 38 of 66 points for the upper extremity). Veterans with multiple strokes were included in this study that lasted for 36 weeks: a 12-week intervention followed by a follow-up period lasting 6 months. Veterans were randomly assigned to the robotic therapy group (RT), N = 49; the intensity-matched comparison group (ICT), N = 50; and the usual care group (UC), N = 28. VA ROBOTICS compared the efficacy of RT to UC and ICT. Usual care was not dictated or prescribed by the protocol. The treatment was allowed to vary per therapy targeting specifically the upper extremity, which consisted of an average of 3 sessions per week from therapists delivering treatment they deemed clinically appropriate for the upper extremity. The RT received 3 sessions per week of robotic training for the shoulder and elbow, wrist, and hand that delivered 1024 movements per session. The ICT received 3 sessions per week of a therapy created to have a therapist deliver comparable movement intensity and repetition to the RT during the same period. Contrary to other rehabilitation studies that used a control intervention expected to have little effect on the primary outcome, VA ROBOTICS was unique in that it included an active control treatment group. The study was based on the hypothesis that RT would experience greater improvement in motor impairment at 12 weeks compared with UC and ICT, as measured by the upper extremity component of the Fugl-Meyer scale. The ICT intervention is not conventional therapy. It uses manual techniques but would likely be impractical to implement as clinical therapy. It is unlikely that therapists could consistently assist the paretic arm during standard clinical care for approximately 1000 movements per session as done for the ICT (instead of the typical 45 movements per session in usual care for chronic stroke patients ). The authors created this control treatment specifically to afford an objective cost analysis.
Results
The first and perhaps most understated finding of the VA ROBOTICS was that usual care did not reduce impairment or disability or improve quality of life in chronic stroke survivors. The usual care intervention had no measurable impact and, to conserve financial resources, it was discontinued as futile midway through the study.
The comparison between the RT and UC included (1) the comparison between the RT and UC subjects, which involved approximately only the first half of the RT when the UC was not discontinued, and (2) whether the changes were robust and long lasting. On this score, robot therapy was statistically superior to usual care in Stroke Impact Scale (quality of life) at the completion of the intervention and in the Fugl-Meyer Assessment (impairment) and Wolf Motor Function Test (function) 6 months after the completion of the intervention.
The results are far more impressive if the whole RT is compared with the UC and not just the analysis that focused on the first half of the study. Although the results at 12 weeks showed that the difference between the first half of the RT and UC was more than 2 Fugl-Meyer points, the difference between the complete RT and UC was 5 points in the Fugl-Meyer assessment, which corresponds to a minimum clinically important difference (MCID) in chronic stroke ( Fig. 1 ).
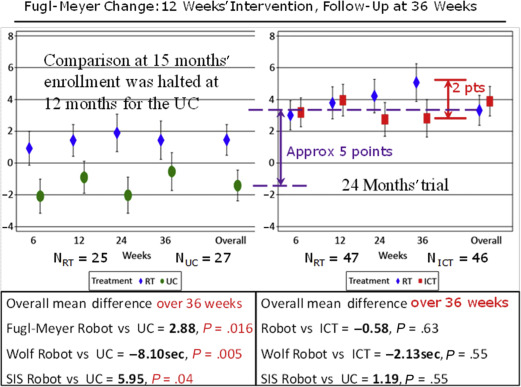
The reason(s) for the smaller clinical effects of the robotic intervention in the first stage of the study compared with the second stage of the study have not been established. The authors think this discrepancy is most likely due to the omission of a phase-in stage in this study. When testing a new therapy, it is common practice to treat a predetermined number of subjects during the initial phase of the trial with the new therapy at each site before beginning data collection for the actual controlled trial to gain familiarity and expertise with the novel treatment and streamline the process. Nevertheless, even without a phase-in stage, VA ROBOTICS demonstrates the robustness of the results: even when therapists are learning how to use the novel tools and cannot deliver the complete protocol in the prescribed period, the results are better than usual care.
The comparison between the RT and ICT did not show any difference. Also patients in the RT continued to improve even after the intervention was completed at 12 weeks. Thus, the continued and persistent improvement at the 6-month follow-up evaluation suggests improved robustness and perhaps an incremental advantage that prompted further improvement even without intervention. For example, an improvement of approximately 3 points in the Fugl-Meyer scale might enable a severe patient to start to raise his/her arm and to bathe independently or to start to stretch the formerly paralyzed arm so that independent dressing could take place. It might enable a more moderate stroke patient to start to tuck in the shirt or to hike the pants independently or to start to reach overhead and actively grasp an object.
This continued improvement after completion of the intervention is remarkable because VA ROBOTICS included patients with chronic stroke disability in the moderate to severe range and more than 30% had multiple strokes. As such, the majority of this group represented a spectrum of disability burden that many studies have avoided. Moreover, 65% of the volunteers interviewed were enrolled. Together these observations suggest that robotic therapy for the upper extremity offers an opportunity to a broad spectrum of stroke patients.
Cost outcomes
In this era of cost containment, an important and unexpected result arose from the recently completed cost-benefit analysis. The purchase cost of the 4 InMotion robotic modules (shoulder and elbow, wrist, antigravity, and hand [Interactive Motion Technologies, Watertown, Massachusetts] Fig. 2 ) was $230,750; the interest rate on borrowing to purchase these robots was estimated at 6.015% with 33% facility overhead on top of the purchase value and a $5000 annual maintenance fee per robot. Yet, the additional costs of delivering RT or ICT were $5152 and $7382, respectively, and the difference was statistically significant ( P <.001). Although the active interventions (RT and ICT) added cost, when total cost was compared, which includes the clinical care needed to take care of these veterans for the 36 weeks of the trial (12 weeks of intervention and 6 months without any active intervention), there were no differences between active intervention and usual care. The total cost for the VA was approximately the same: $17,831 for RT; $19,746 for ICT; and $19,098 for UC. The UC used the rest of the health care system more often than the active intervention groups. For 36 weeks of care, the RT cost the VA $5152 for robotic therapy and $12,679 for clinical care. For 36 weeks of care, the UC cost the VA approximately $19,098.











