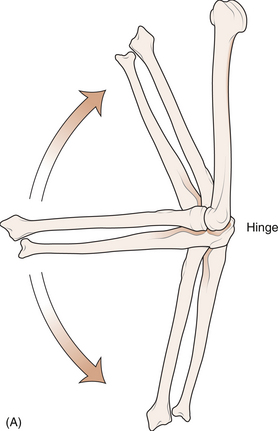
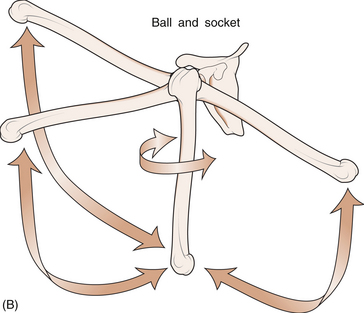
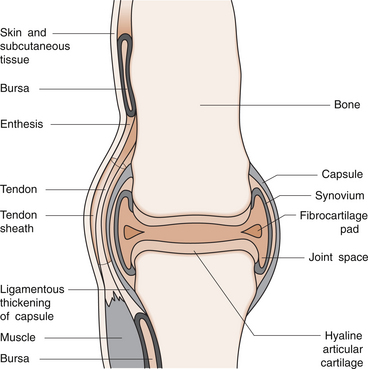
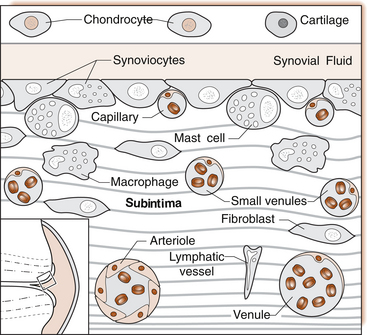
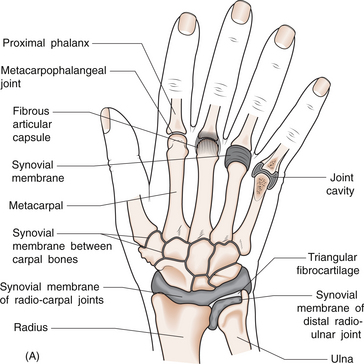
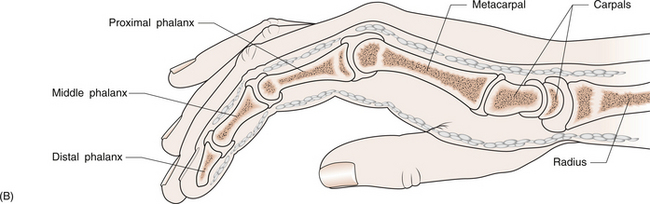
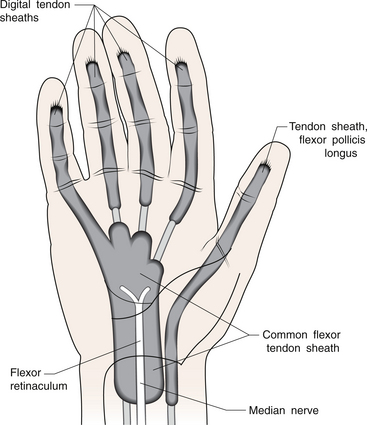
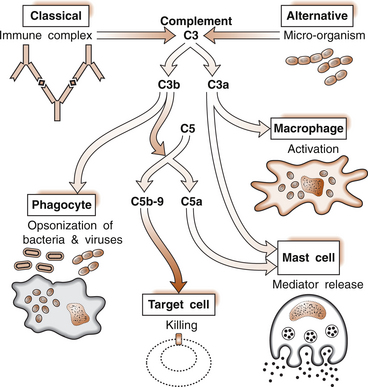
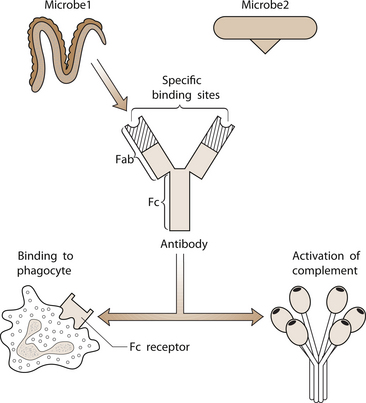
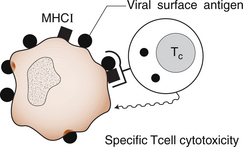
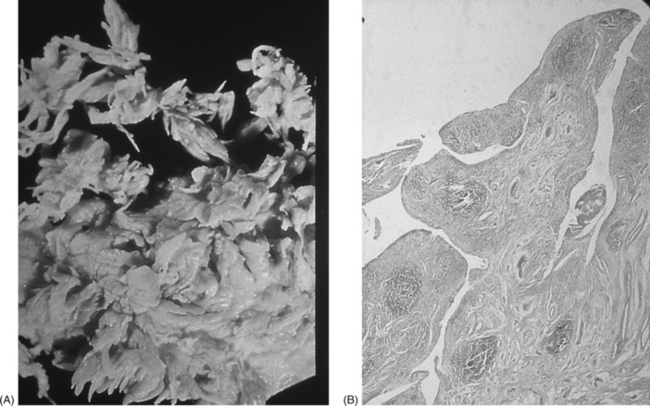
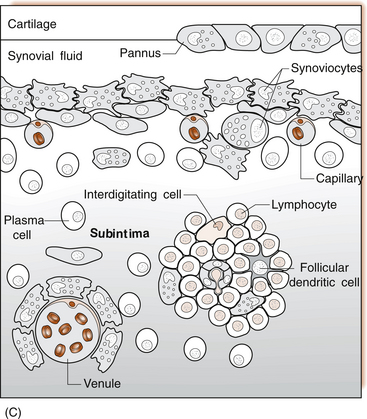
1 Leslie Schrieber Synovial, or diarthrodial joints, are the commonest type of joint and are the most mobile. They possess a synovial membrane, have a cavity that contains synovial fluid, and are subclassified into ball and socket (e.g. hip), hinge (e.g. interphalangeal) and saddle (e.g. first carpometacarpal) types. These joints (Fig. 1.1) allow the cartilaginous surfaces of the joint ends to move efficiently and smoothly, with low frictional resistance. Different designs allow for different movements, including flexion (bending), extension (straightening), abduction (movement away from midline), adduction (movement towards midline), and rotation. They are more susceptible to inflammatory injury than are other types of joints. Synovial joints are surrounded by a capsule that defines the boundary between articular and periarticular structures (Fig. 1.2). Reinforcing the capsule are ligaments and muscular tendons, which act across the joint. The joint capsule, ligaments and tendons are composed principally of type 1 collagen fibres—type 1 collagen is the major fibrous protein of connective tissue. The synovium has a lining layer that consists of special cells called synoviocytes and is normally one to three cells thick. There is no basement membrane separating the synoviocyte layer from the subintima (Fig. 1.3). There are at least two different types of synoviocyte cell: type A and type B. Type A are of bone marrow-derived macrophage (phagocyte or ‘hungry cell’) lineage and type B are fibroblast-like mesenchymal (connective tissue) cells. Other cell types in this layer include dendritic cells—antigen-processing cells involved in generating an immune response. The synoviocytes lie in a stroma composed of collagen fibrils and proteoglycans (a diverse group of glycosylated proteins that are abundant in the extracellular matrix of connective tissues), which is continuous with the subintima. The latter may be fibrous, fatty or areolar (contain loose connective tissue). It contains a dense network of fenestrated capillaries (small blood vessels) that facilitate the exchange of molecules between the circulation and the synovium. The vessels are derived from branches of the arterial plexus that supplies the joint capsule and juxta-articular bone. There is also a lymphatic supply—a vascular system involved in removing large molecules from the synovium. The latter is innervated and pain sensitive, particularly during inflammation. Synovial fluid (Fig. 1.4) is present in small quantities in normal synovial joints. It is a clear, relatively acellular, viscous fluid that covers the surface of synovium and cartilage. Synovial fluid is an ultrafiltrate of blood to which hyaluronic acid is added. Hyaluronic acid is secreted by synoviocytes and is the molecule responsible for synovial fluid viscosity, acting as a lubricant for synovial–cartilage interaction. Synovial fluid represents an important site for exchange of nutrients and metabolic by-products between plasma and the surrounding synovial membrane. The synovial cavity can be used to advantage as a site in which therapeutic agents are introduced, e.g. intra-articular corticosteroids to treat inflamed synovium, as well as for diagnostic aspiration. The proximal and distal interphalangeal joints are true hinge joints whose movements are restricted to flexion and extension. Each joint has a thin dorsal (upper surface) capsular ligament strengthened by expansion of the extensor tendon, a dense palmar (under surface) ligament, and collateral ligaments on either side of the joint. The metacarpophalangeal joints are also considered hinge joints and their ligaments resemble those of the interphalangeal joints. When the fingers are flexed, the heads of the metacarpal bones form the rounded prominences of the knuckles, with the joint space lying about 1 cm distal (peripheral) to the apices of the knuckles. Figure 1.5 shows the relationship of the dorsal and lateral aspects of the joint space, synovial membrane and the articular capsule to adjacent structures. The wrist or radiocarpal joint is formed proximally by the distal end of the radius and the articular disc, and distally by a row of carpal bones, the scaphoid, lunate, pisiform and triquetrum (Fig. 1.5A). The articular disc joins the radius to the ulnar and separates the distal end of the ulnar from the wrist joint proper. The wrist joint is surrounded by a capsule and supported by ligaments. The distal radioulnar joint is adjacent to but not normally part of the wrist joint, since the articular disc divides these joints into separate cavities (Fig. 1.5A). The midcarpal joint is formed by the junction of the proximal and distal rows of the carpal bones. Limited flexion, extension and some rotation occur in the midcarpal joint. Pronation and supination occur primarily at the proximal and distal radioulnar articulations. The long flexor tendons of the muscles of the forearm are enclosed in a common flexor tendon sheath that begins proximal to the wrist crease and extends to the midpalm (Fig. 1.6). Part of the common flexor tendon sheath lies in the carpal tunnel and is bounded anteriorly by the flexor retinaculum (a ligament that lies on the volar surface of the wrist). Thickening of the synovial membrane of the flexor tendons because of synovitis can cause carpal tunnel syndrome (see Ch. 3). Innate defence mechanisms include the protective effects of intact skin and mucosa in combating microbes. Normal skin acts as an impermeable barrier to most infectious agents. Mucus secreted by the membranes lining the inner surfaces of the body (e.g. nasal and bronchial mucosa) acts as a protective barrier that prevents bacteria adhering to epithelial cells. Another innate line of defence against microbes is the complement system. This comprises over 20 proteins. The complement system is able to respond rapidly to a trigger stimulus, resulting in activation of a sequential cascade in which one reaction is the enzymatic catalyst of the next (Fig. 1.7). The most important complement component is C3, which facilitates the uptake and removal of microbes by enhancing their adherence to the surface of phagocytic cells. Biologically active fragments of C3–C3a, and C5a are able to attract PMNs (called chemotaxis) as well as activating these cells. Activated complement components later in this sequence, C6, 7, 8 and 9, form a complex—the membrane attack complex—on the surface of target cells and this is able to punch holes in the cell membrane, resulting in target cell lysis. Antibodies are remarkable proteins produced by bone- marrow derived B lymphocytes, which are able to differentiate into plasma cells. Antibodies are adaptor molecules that are capable of binding to phagocytic cells, activating complement and binding to microbes. Each antibody has a unique recognition site for a particular microbe—the Fab end of the molecule, which binds microbes (Fig. 1.8). Molecules in the microorganism that evoke and react with antibodies are called antigens. The Fc end of the antibody molecule contains domains capable of binding and activating the first component of complement as well as binding to phagocyte Fc receptors. There are five antibody subtypes, classified by variations in the structure of the Fab region: IgG, IgM, IgA, IgD and IgE. The ability to recognize a particular antigen and distinguish it from a different antigen is related to the ability to distinguish between self-antigen and non-self (i.e. foreign) antigens. There is an active process by which self-antigen fails to induce an immune response, known as tolerance. In some circumstances, tolerance is broken and the individual produces self-directed antibodies known as autoantibodies. These may give rise to autoimmune diseases. Another autoimmune disease, systemic lupus erythematosus, is discussed in Chapter 9. There is also another subpopulation of T lymphocytes, known as cytotoxic T cells, which recognize antigen expressed on the surface of target cells in association with MHC class 1 molecules (Fig. 1.9). The cytotoxic T cell comes into direct contact with the target cell and kills it. Just as is true for B cells, T cells selected and activated by binding antigen undergo clonal proliferation and mature to produce T helper and cytotoxic cells and produce memory cells. The latter can be reactivated upon further antigenic challenge. In the early stages of RA, the synovium becomes oedematous (contains excess fluid), thickened, hyperplastic (cells multiply excessively) and develops villus-like projections as found in normal small intestine (Fig. 1.10A). The synovial lining layer undergoes cellular proliferation and becomes multilayered. One of the earliest histological changes is injury to the synovial microvasculature, with swelling of endothelial cells, widened interendothelial gaps and luminal occlusion. There is dense synovial cellular infiltration with prominent perivascular T lymphocytes, plasma cells and macrophages, but few neutrophils (Fig. 1.10B). Prominent fibrin deposition is characteristic. Lymphoid nodular aggregates composed principally of CD4 T (helper) cells may be found in the synovial stroma (Fig. 1.10C), but are more likely to develop later in the disease. By contrast, in the synovial fluid there is a predominance of neutrophils. RA often involves periarticular structures including tendon sheaths and bursae.
RHEUMATOID ARTHRITIS AND THE HAND
Essential anatomy and physiology
Synovial joint anatomy
Synovial joint physiology
Anatomy of the hand and wrist joints
Joints and synovial membranes
Tendons
Essential immunology
Innate mechanisms
Antibodies
Cell-mediated immunity
Pathology
Synovitis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


RHEUMATOID ARTHRITIS AND THE HAND
Only gold members can continue reading. Log In or Register to continue