Rheumatic Fever
Galal M. El-Said
Yasser M. K. Baghdady
Howaida G. El-Said
Rheumatic fever (RF) is a delayed, nonsuppurative sequela to upper respiratory infection with group A beta-hemolytic streptococci. It is a diffuse inflammatory disease of the connective tissue that involves principally the heart, blood vessels, joints, central nervous system, and subcutaneous tissues.
The term acute RF is a misnomer because it may not be acute, rheumatic, or febrile. Although the term emphasizes involvement of the joints, the disease owes its importance to involvement of the heart. As early as 1884, Lasegue described this feature: “Rheumatic fever is a disease that licks the joints but bites the heart.”
EPIDEMIOLOGY
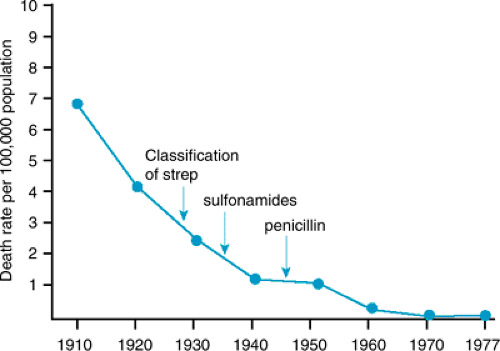
Numerous investigators have documented the declining incidence of RF, even before the introduction of penicillin (Fig. 285.1). In Rochester, Minnesota, the age-adjusted annual incidence per 100,000 persons was 20 from 1939 to 1949, 12 from 1950 to 1964, and 3 from 1965 to 1978. Several more recent outbreaks in military and civilian populations in the United States correlated with the reappearance of virulent M-protein strains belonging to the M serotype associated with previous RF epidemics.
In contrast to trends in the United States, the incidence of RF has not decreased in developing countries. Worldwide, an estimated 5 to 30 million children and young adults have chronic rheumatic heart disease (RHD), and 90,000 patients die of this disease each year.
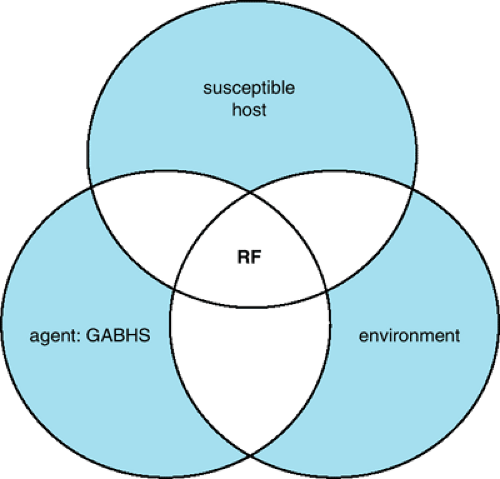
Several factors predispose to development of RF: age, family history, season, recurrent streptococcal infections, and host factors affecting susceptibility. The first attack usually occurs in patients between 5 and 15 years of age. RF is a a rare occurrence in children younger than 4 years of age. No gender preference exists unless chorea is included, in which case the incidence is slightly greater among girls.
RF may affect more than one member in the same family. The same housing conditions possibly predispose several persons to recurrent streptococcal infections. Constitutional susceptibility may be a factor, but no evidence for genetic markers in rheumatic patients exists, and HLA genotypes do not correlate with the development of RF. Like streptococcal pharyngitis, RF occurs more commonly in the winter and spring.
Recurrent streptococcal infections are the most important predisposing factor in the occurrence and recurrence of RF. Approximately 1% to 5% of streptococcal throat infections are followed by development of RF. The most important factors that may be related to the attack rate of RF after streptococcal pharyngitis are the magnitude of the immune response to the antecedent infections and the duration of convalescent carriage of the organisms. Skin infections are unlikely to produce the disease.
Because RF develops after streptococcal pharyngitis in a relatively small percentage of patients, host predisposition probably is a factor (Fig. 285.2). After RF is acquired, its reactivation after subsequent streptococcal infections is much more likely to occur. The recurrence rate per infection is approximately 50% during the first year after the initial attack; it decreases sharply after that. The rate levels off after several years to
approximately 10%. This persistently high attack rate after RF suggests acquired hyperreactivity.
approximately 10%. This persistently high attack rate after RF suggests acquired hyperreactivity.
ETIOLOGY
Streptococci
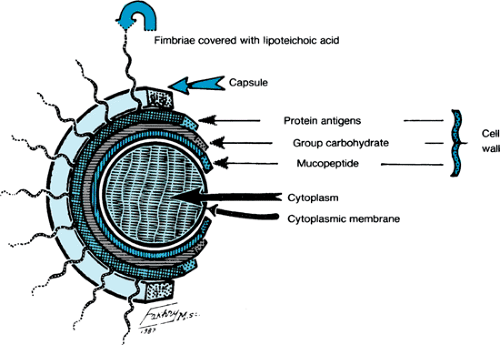
Streptococci are a large group of gram-positive microorganisms that are distributed widely in nature. When cultured, they are arranged in chains. Their ability to hemolyze erythrocytes to various degrees is an important basis for their classification. The alpha-hemolytic streptococci form colonies surrounded by an ill-defined, greenish halo in which hemolysis is incomplete. The beta-hemolytic streptococci produce a clear zone of hemolysis on blood agar; gamma-streptococci do not have any effect on blood-containing media. The streptococcus (Fig. 285.3) is composed of a core of cytoplasm surrounded by three layers: a cytoplasmic membrane, a cell wall, and a capsule that constitutes the external surface of the organism. The capsule is composed of hyaluronate, which is nonantigenic. The cell wall is composed of three layers: the outermost protein, the middle carbohydrate, and the innermost mucopeptide protoplast. The middle layer of the cell wall, the carbohydrate layer, provides the group specification, given as the alphabetic classification (e.g., A through H) of Lancefield. The outer protein layer and its fimbriae, which are attached to the surface of the organism, contain the proteins designated M, T, and R. The M protein is the most important because it determines the virulence of the organism, stimulates formation of opsonizing and precipitating antibodies, and may impede phagocytosis. Lancefield and colleagues further classified group A streptococci into serologic types on the basis of the M protein. RF can result from infection by many of the serotypes of group A beta-hemolytic streptococci, but glomerulonephritis is associated with only a limited number of these serotypes. The cytoplasmic membrane is an antigenic lipoprotein that cross-reacts with several mammalian tissue antigens, including the glomerular basement membrane and sarcolemmal antigen.
The streptococcus produces several extracellular products, some of which are involved in the diseases produced by the microorganism. Erythrogenic toxin is responsible for the rash of scarlet fever. Streptolysin O, which is active only in the reduced state, is cardiotoxic and leukotoxic, is responsible for hemolysis of erythrocytes, and elicits an antibody response, antistreptolysin O (ASO), which is the basis for a useful assay of streptococcal infections. The antigenicity of streptolysin O is inhibited by lipid extracts (probably cholesterol) of skin, and this property may be responsible for the lack of association between streptococcal skin infections and RF. Streptolysin S, an oxygen-stable product, produces the hemolysis characteristic of beta-hemolytic streptococci when cultured on sheep blood agar. Streptokinase converts plasminogen to plasmin, an active proteolytic enzyme that digests fibrin. Diphosphopyridine nucleotidase, which determines the ability of the organism to kill leukocytes, elicits an antibody response (i.e., antidiphosphopyridine nucleotidase). There are four deoxyribonucleases (i.e., A, B, C, D), all of which are antigenic. Deoxyribonuclease B, known as streptodornase, is produced in the largest quantities in response to group A streptococcal infections and is the most consistent of the deoxyribonucleases.
RF develops when children or adolescents develop pharangitis with group A beta-hemolytic streptococcal infection. The organism attaches itself on the epithelium of the upper respiratory tract and invades the tissues. The incubation period is 2 to 4 days, and the invading organism elicits an acute inflammatory response resulting in sore throat, fever, malaise, headache, and an elevated leukocyte count that last for 3 to 5 days. RF occurs in a small percentage of these patients after resolution of the sore throat. Only infections of the pharynx initiate or reactivate RF.
Direct contact with respiratory secretions transmits the organism, and crowding enhances transmission. Patients remain infected for weeks after symptomatic resolution of pharangitis and may serve as a reservoir for infecting others.
Mechanism Producing Rheumatic Fever
RF is thought to be an autoimmune disease. The requirements for its development include group A beta-hemolytic streptococcal infection in the throat with an antibody response indicative of recent infection and persistence of the organism in the pharynx for a period sufficient to produce an immunologic
response. The magnitude of the antibody response is a major factor determining the attack rate of RF after streptococcal infection. The predisposing organism has antigens immunologically similar to proteins in the human heart, and the antibodies produced against the streptococci react with the heart (i.e., cross-reactive immunity). A plethora of streptococcal cellular components cross-reacting with various mammalian tissues has been described. The hyaluronate capsule is identical to human hyaluronate. Antibodies to the cell wall polysaccharide cross-react with glycoproteins of heart valves. Membrane antigens cross-react with the sarcolemma and smooth muscles of endocardial and myocardial arteries. Antibody to the streptococcal group A polysaccharide persists in the serum of patients with rheumatic valvular disease, in contrast to its more rapid decline in patients with RF without cardiac involvement. As with other possible autoimmune diseases, the difficulties of differentiating cause from effect of injury have rendered this hypothesis unprovable.
response. The magnitude of the antibody response is a major factor determining the attack rate of RF after streptococcal infection. The predisposing organism has antigens immunologically similar to proteins in the human heart, and the antibodies produced against the streptococci react with the heart (i.e., cross-reactive immunity). A plethora of streptococcal cellular components cross-reacting with various mammalian tissues has been described. The hyaluronate capsule is identical to human hyaluronate. Antibodies to the cell wall polysaccharide cross-react with glycoproteins of heart valves. Membrane antigens cross-react with the sarcolemma and smooth muscles of endocardial and myocardial arteries. Antibody to the streptococcal group A polysaccharide persists in the serum of patients with rheumatic valvular disease, in contrast to its more rapid decline in patients with RF without cardiac involvement. As with other possible autoimmune diseases, the difficulties of differentiating cause from effect of injury have rendered this hypothesis unprovable.
Conclusion
Although the precise pathogenetic mechanisms of RHD remain elusive, a mounting body of evidence is emerging to support the concept of an abnormal, autoimmune host response following exposure of susceptible individuals to group A streptococcal antigens. Abnormalities in both cellular and humoral immune responses have been documented in affected patients.
PATHOLOGY
General Pathology
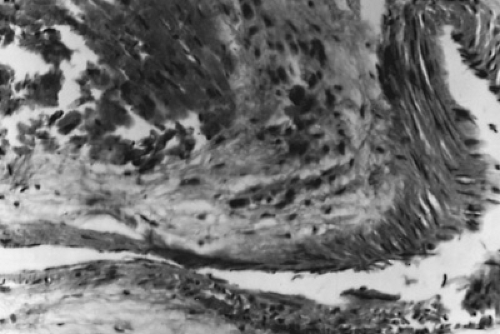
Nonspecific lesions result from fibrinoid degeneration of the connective tissue, inflammatory edema, and inflammatory cell infiltration. Specific lesions result from a proliferative reaction that forms Aschoff nodules (Fig. 285.4). Aschoff nodules are paravascular nodules consisting of a center of fibrinoid degeneration surrounded by Aschoff cells, lymphocytes, and fibroblasts.
Cardiac Pathology
RF affects the three layers of the heart, causing endocarditis, myocarditis, and pericarditis (i.e., pancarditis). Endocarditis affects the mitral and aortic valves and rarely affects the tricuspid or pulmonary valves. In the active phase, the valves become edematous and the endocardium is damaged along the contact margins 2 or 3 mm from the free edges. At this site, tiny vegetations consisting of platelet thrombi are formed (Fig. 285.5). After inflammation subsides, fibrosis and contracture occur. Similar changes cause shortening of the chordae tendineae. The site of the lesions, at the contact points of the cusps, suggests that trauma determines the position of the damage. The degree of trauma determines the frequency with which the valves are involved. The mitral valve, closing at the highest pressure (greater than120 mm Hg), is subjected to the greatest stress and is the valve most frequently affected. The aortic valve, which closes by systemic diastolic pressure of 80 mm Hg, is the valve next most often involved, followed by the tricuspid valve and the pulmonary valve. When the inflammation is severe, the cusps are damaged, and insufficiency develops. Mild inflammation thickens tissue, and adhesions occur between the cusps. The adhesions gradually increase, producing stenosis of the valve orifice.
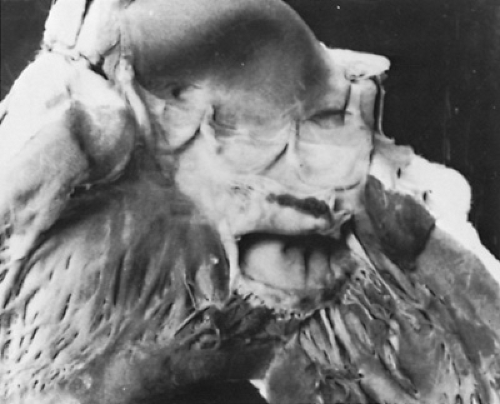 FIGURE 285.5. An opened left ventricle displays the mitral and aortic valves of a patient with acute rheumatic fever. The mitral valve shows small (1- to 2-mm) linear vegetations on the atrial surface of the anterior and posterior mitral leaflet margins. The aortic valve appears to be unaffected. (Courtesy of Dr. Soheir Mahfouz, Pathology Department, Cairo University, Cairo, Egypt.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|