Abstract
Implanted phrenic nerve stimulation is a technique restoring spontaneous breathing in patients with respiratory control failure, leading to being dependent on mechanical ventilation. This is the case for quadriplegic patients with a high spinal cord injury level and for patients with congenital central hypoventilation syndrome. The electrophysiological diaphragm explorations permits better patient selection, confirming on the one hand a definite issue with central respiratory command and on the other hand the integrity of diaphragmatic phrenic nerves. Today there are two different phrenic stimulation techniques: the quadripolar intrathoracic stimulation and the bipolar intradiaphragmatic stimulation. Both techniques allow patients to be weaned off their mechanical ventilator, improving dramatically their quality of life. In fact, one of the systems (phrenic intradiaphragmatic stimulation) was granted social security reimbursement in 2009, and now both are reimbursed. In the future, phrenic intradiaphragmatic stimulation may find its place in the intensive care unit, for patients needing it temporarily, for example, after certain surgeries with respiratory complications as well as diaphragmatic atrophies induced by prolonged mechanical ventilation.
Some neurological pathologies render patients dependent on mechanical ventilation because of a defect in respiratory command or respiratory command transmission (“central respiratory paralysis”), while the main respiratory effector (the diaphragm) remains intact. This dependence on mechanical ventilation leads to a loss of autonomy and can make the return home quite difficult for these patients. Implanted phrenic stimulation is a therapeutic approach allowing patients to wean off mechanical ventilation.
Historically, the first stimulation of the phrenic nerve dates back from the discovery of electricity. The first description of diaphragmatic contraction induced by phrenic stimulation at neck level dates back to 1819, on the “fresh” cadaver of a hanged prisoner . Right from 1829, Jean-Jacques Leroy from Etiolles, after having described the dangers of too much positive pressure insufflation, recommended calibrating this insufflation based on the weight of the subject, he underlined that the most natural mechanical ventilation was the one that could be induced by stimulating the diaphragm. He put the concept into practice, using needles inserted in the diaphragm in a cat model . Duchenne de Boulogne described in details the technique of phrenic stimulation at neck level and suggested therapeutic applications, in the rehabilitation of lead poisoning paralysis for example . In the modern era, the first clinical applications of implanted phrenic stimulations started in the seventies . In France, the first activity in this area was noted in the eighties, by a team from the Berck PM&R center .
The dependence of mechanical ventilation for quadriplegic patients following a high level spinal cord injury (SCI) is the most frequent indication for implanted phrenic stimulation . Other indications (congenital or acquired central hypoventilation syndrome) are rarer. We estimate that around 50 patients per year might be concerned by this indication in France. Two techniques are available today intrathoracic and intradiaphragmatic phrenic nerve stimulation (see the details in the report published in 2009 by the French Higher Health Authority [HAS], 2009). Today, both techniques are reimbursed by the French social security system.
The objectives of this literature review is to cover today’s main indications and contraindications, detail the two available techniques and refine the different therapeutic perspectives where applications pertaining to intensive care reanimation could be relevant in years to come.
1
Reminder: physiology and pathophysiology of respiratory command
The mobilization of lung volumes, necessary for gas exchanges ensuring respiratory function, is under the dependence of motor pressures produced by respiratory muscles ( Fig. 1 ). The activity of these muscles must be maintained all lifelong 24/24, even when sleeping. The command of these muscles is intrinsic, it stems from the central nervous system (CNS). This respiratory command is a dual one, automatic at the level of the brain stem and voluntary at cortical level. The command is transmitted to the spinal motor neurons dedicated to respiratory muscles via the bulbospinal and corticospinal tracts. The automatic motor command sources from the brain stem at the level of the pre-Bötzinger complex (preBötC) and the parafacial zone. This command is exclusive during sleep and is permanently active. It is submitted to numerous mechanical and metabolic afferents but also supraspinal influences, coming from example from the limbic system.
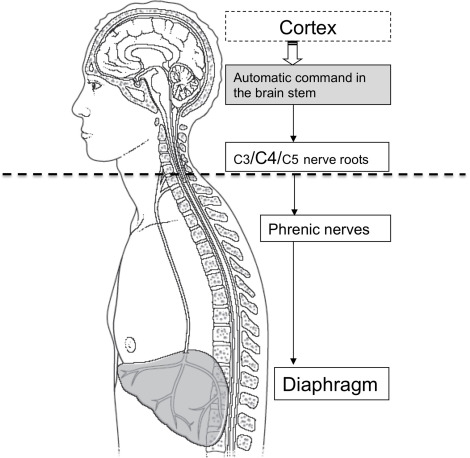
The voluntary respiratory command stems from the cortical structure belonging to the locomotor system. This command enables voluntary respiratory maneuvers, such as apnea or respiratory functional tests.
Motor neurons dedicated to the motor innervation of the diaphragm form, with the sensory afferents coming from the diaphragm, the phrenic nerves . They stem from the anterior part of the anterior grey column (C3, C4 and C5 levels).
The C4 nerve root is considered as the main root (75% of fibers make up the phrenic nerve). Fibers stemming from these three roots rapidly unite to make up the phrenic nerve at a height corresponding approximately to the transversal plane going through the upper rim of the thyroid cartilage where it is covered by the sternocleidomastoid muscle, it follows a downstream vertical path. The phrenic nerve receives the accessory phrenic nerve (stemming from C5) in its supraclavicular portion.
In the thorax, the phrenic nerve, plastered against the pleura, goes down all the way to the diaphragm in the anterior mediastinum, bordering the large vessels from the base of the heart, then the lateral side of the pericardium. In the mediastinum, both nerves follow a different path: the right one goes straight in a straight path, vertically, along the superior vena cava and the right atrium, it approaches the diaphragm on the anteroexternal side of the inferior vena cava. The left one, which is longer, describes a wide curve at the left side of the heart and thus approaches the diaphragm, a little bit backward of the tip of the heart, in a more anterior and external approach. Both nerves divide in their terminal branches at the level of the inferior surface (abdominal) of the diaphragm. There are generally 3 terminal branches.
Thus, the diaphragmatic function can be compromised at three different levels ( Fig. 1 ):
- •
at the level of central command or the upper motor neuron;
- •
at the level of the peripheral phrenic motor neuron or along the path of the phrenic nerve in the thorax;
- •
at muscle level.
Implanted phrenic stimulation is then relevant in its validated indications, presuming the phrenic nerve is intact and there is no irreversible or progressive diaphragm atrophy. In this regard, these indications are limited to respiratory central command disorders .
2
Surgical techniques
Two phrenic stimulation techniques are nowadays available in France.
2.1
Intrathoracic phrenic stimulation
Intrathoracic phrenic stimulation is the “historical” technique ( Fig. 2 ) . It is based on radiofrequency transmission via transmitter coils fixed to the skin, to a receptor implanted under the skin linked to phrenic electrodes, energy and information produced and modulated by an external stimulator (Atrostim ® , Atrotech, Tampere, Finland or Avery ® , Avery medical System, USA). The stimulation is bipolar (Avery ® ) or quadripolar and sequential (Atrostim ® ). Electrodes are implanted, via a minimally invasive thoracotomy, on each phrenic nerve (above the superior vena cava for the right phrenic nerve, and above the pulmonary trunk for the left phrenic nerve). It is essential to control the proper functioning of the electrodes during the surgery, by determining the stimulation threshold of each electrode, which must be inferior to 1 mA.
2.2
Intradiaphragmatic phrenic stimulation
This implantation technique is more recent (NeurRxDP4 ® , Synapse, Oberlin, Ohio, USA) ( Fig. 3 ). Stimulation electrodes are implanted during laparoscopy, after mapping via stimulation during surgery in order identify the phrenic motor point in each diaphragmatic cupola, two stimulation electrodes are positioned at this level. Thus, the phrenic nerve is stimulated in its distal part, via two intradiaphragmatic electrodes. The electrodes are then tunneled under the skin and connected to the phrenic stimulator via a connector and a cable.
2.3
Advantages and drawbacks
Intrathoracic phrenic stimulation is the gold standard with multiple patients implanted all over the world for nearly 40 years. It guarantees the stimulation of all phrenic fibers, with stimulation intensities ranging from 1 to 2 mA. It implies nevertheless, a rather complicated surgery, requiring a thoracotomy, with a potential risk of phrenic nerve lesion during surgery and a postoperative period that includes more potential complications than laparoscopy . This is a costly technique, an Atrostim ® stimulator from Atrotech (Tampere, Finland) costs about 50,000 €. The intradiaphragmatic system (Synapse Biomedical, Oberlin, Ohio, USA) requires a less invasive surgery with the absence of phrenic nerve lesion risks. This device costs less than 20,000 €. This approach does not guarantee the stimulation of all phrenic fibers, and requires high stimulation intensities, which can reach 25 mA. Its effectiveness can be compromised, in patients with no damages to sensory pathways (congenital or acquired hypoventilation, some incomplete spinal cord injuries, by pain projected at shoulder level triggered by the stimulation of sensory phrenic afferents . This pain can limit the efficacy of stimulation and might require the use of analgesics targeting neuropathic pain.
2
Surgical techniques
Two phrenic stimulation techniques are nowadays available in France.
2.1
Intrathoracic phrenic stimulation
Intrathoracic phrenic stimulation is the “historical” technique ( Fig. 2 ) . It is based on radiofrequency transmission via transmitter coils fixed to the skin, to a receptor implanted under the skin linked to phrenic electrodes, energy and information produced and modulated by an external stimulator (Atrostim ® , Atrotech, Tampere, Finland or Avery ® , Avery medical System, USA). The stimulation is bipolar (Avery ® ) or quadripolar and sequential (Atrostim ® ). Electrodes are implanted, via a minimally invasive thoracotomy, on each phrenic nerve (above the superior vena cava for the right phrenic nerve, and above the pulmonary trunk for the left phrenic nerve). It is essential to control the proper functioning of the electrodes during the surgery, by determining the stimulation threshold of each electrode, which must be inferior to 1 mA.
2.2
Intradiaphragmatic phrenic stimulation
This implantation technique is more recent (NeurRxDP4 ® , Synapse, Oberlin, Ohio, USA) ( Fig. 3 ). Stimulation electrodes are implanted during laparoscopy, after mapping via stimulation during surgery in order identify the phrenic motor point in each diaphragmatic cupola, two stimulation electrodes are positioned at this level. Thus, the phrenic nerve is stimulated in its distal part, via two intradiaphragmatic electrodes. The electrodes are then tunneled under the skin and connected to the phrenic stimulator via a connector and a cable.
2.3
Advantages and drawbacks
Intrathoracic phrenic stimulation is the gold standard with multiple patients implanted all over the world for nearly 40 years. It guarantees the stimulation of all phrenic fibers, with stimulation intensities ranging from 1 to 2 mA. It implies nevertheless, a rather complicated surgery, requiring a thoracotomy, with a potential risk of phrenic nerve lesion during surgery and a postoperative period that includes more potential complications than laparoscopy . This is a costly technique, an Atrostim ® stimulator from Atrotech (Tampere, Finland) costs about 50,000 €. The intradiaphragmatic system (Synapse Biomedical, Oberlin, Ohio, USA) requires a less invasive surgery with the absence of phrenic nerve lesion risks. This device costs less than 20,000 €. This approach does not guarantee the stimulation of all phrenic fibers, and requires high stimulation intensities, which can reach 25 mA. Its effectiveness can be compromised, in patients with no damages to sensory pathways (congenital or acquired hypoventilation, some incomplete spinal cord injuries, by pain projected at shoulder level triggered by the stimulation of sensory phrenic afferents . This pain can limit the efficacy of stimulation and might require the use of analgesics targeting neuropathic pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








