Fig. 11.1
Dynamic stepping lunges. The lunge begins with slow controlled eccentric loading (a), followed by explosive concentric contraction to return to the starting position (b)

Fig. 11.2
Single-limb lunge stance with lateral reach
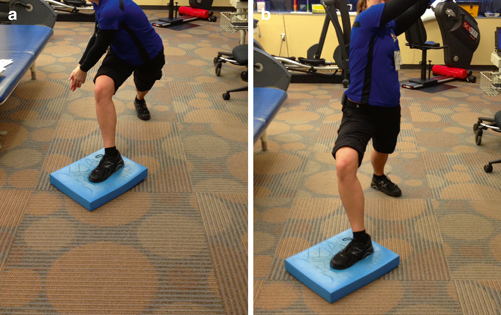
Fig. 11.3
Single-limb stance and reaching with contralateral trunk rotation
Neurovascular Entrapment
Neurovascular entrapment , including diagnoses such as PAES, can occur when the popliteal artery and other neurovascular structures become compressed between muscles and tendons in the posterior lower leg due to variations in embryonic development [20]. The main reason for delay in diagnosis of neurovascular entrapment is a lack of consideration for vascular or neurological pathology in patients without cardiovascular risk factors [21].
Use of neurodynamic mobilization as a treatment for neurovascular entrapment has become increasingly popular in the rehabilitation of this disorder. Prolonged compression of a nerve can lead to edema and increased pressure, resulting in additional compression due to intraneural thickening. It has been demonstrated that neurodynamic mobilization techniques can reduce intraneural edema and decrease the effects of compression from surrounding fascial restrictions [34]. Neural mobilization techniques increase excursion by “flossing” the nerve. This technique attempts to mobilize the neural tissue both proximally and then distally [35]. Alternating between elongating and shortening the nerve creates a pumping effect to help decrease edema by dispersing the intraneural fluid [34]. It has been suggested that these mobilization techniques should be performed in a slow and rhythmic fashion to prevent excessive strain, which would in turn inhibit blood flow and further damage the nerve.
It is also important to evaluate the location of the injury and consider joint position during these mobilization techniques. Boyd et al. discuss the effects of proximal–distal sequencing versus a distal–proximal sequencing of joint movement to create neural gliding [36]. The joint closest to the injured site is more irritable due to intraneural edema and, therefore, should remain somewhat stationary, given that the greatest amount of strain occurs at the moving joint [35, 36]. Butler et al. describe varying techniques for passive and active mobilization strategies for the tibial and peroneal nerves [37]. A combination of ankle dorsiflexion and eversion with knee extension and hip flexion provides maximal elongation of the tibial nerve. For the sural nerve, maximal elongation comes from a combination of ankle dorsiflexion and inversion with knee extension and hip flexion [37].
Mobilizing the nerve through surrounding structures is a key component of the rehabilitation process, but consideration of the fascial restrictions causing the entrapment is critical. Addressing these restrictions with myofascial release techniques has been found to be beneficial as a conservative management [37]. Moreover, consideration of why these restrictions may be present through assessment of an anatomical alignment is important in rehabilitation for prevention of recurrence [37]. The final stage of rehabilitation should consist of developing a home exercise program to increase tissue extensibility of restricted muscles and to strengthen weak muscles that may contribute to any postural misalignments [37].
Tibialis Posterior
Tibialis posterior injuries are a very common problem in sports medicine. Although they are rarely reported or studied, posterior tibialis tendon issues are a common cause of acquired adult flat foot, among other problems, and the mechanism of injury has not been clearly elucidated [38].
Musculoskeletal abnormalities can usually be diagnosed by the use of the mnemonic “TART”: Tenderness, Asymmetry, Restricted motion, and Tissue texture abnormality [39]. Dixon et al. described a three-grade classification of calf muscle strains based on disability, physical findings, and pathologic correlation. Although there is consensus about the use of a three-grade classification, there seems to be disagreement in the semantics of the grading system [16]. Johnson and Strom described a classification for tibialis posterior tendon insufficiency classification, later complemented by Myerson [38, 40]. Although this is a grading system for tendon insufficiency and not muscular strain, Howitt et al. considers the three-stage Johnson and Strom system useful for establishing treatment strategies as the acute tibialis posterior strain would be equivalent to a stage 1 or 2 of this classification scale [38].
There seems to be consensus regarding the initial treatment of acute muscle strain. Similar to prior sections of this chapter, most experts agree about the inclusion of the PRICE system for initial management of tibialis posterior muscle strains, as with any other muscle strains [16, 38–40]. Symptom control, avoidance of hemorrhage, and other complications should be the main goals of this phase [16, 38].
Protection from further injury must be one of the initial steps. Only severe injuries require total immobilization and avoidance of weight-bearing [39, 41]. Use of braces or other orthotic devices are particularly useful to correct biomechanical problems, which may hinder optimal healing [42]. Rest with a relative limitation of physical activity, decreased repetitive loading, and cessation of participation in sports for a short period of time as determined by the severity of the symptoms might be needed to achieve symptom control and prevent further injuries [39, 42]. A brief period of immobilization, usually limited to no more than 1 week, is recommended to allow the scar tissue to gain the necessary strength to tolerate the forces applied over the new tissue; this will have the beneficial effect of preventing acute reinjuries and retraction of the muscle stump [43]. Prolonged immobilization should be avoided if possible to prevent muscular atrophy and deconditioning [42].
Cryotherapy by applying ice to the affected area intermittently has been recommended in multiple studies. It has been effective for short-duration pain relief and swelling control. It has also been associated with smaller hematomas between the myofiber stumps [43]. Although there is agreement in the general effectiveness of cryotherapy, there is no consensus regarding the specific duration and interval of the therapy. Some authors recommend intermittent application for a 10 min duration, while others recommend periods of 15–20 min [39, 42, 43]. Recent research suggests the greatest benefit of cryotherapy occurs in the 6 h following the initial trauma [43].
Compression is a component of the PRICE treatment and has been widely recommended in several studies in combination with cryotherapy at similar intervals because it reduces blood flow to the injured fibers. However, there is no conclusive information regarding the use of compression in the acute phase of the injury, and it has not been proven to hasten healing [16, 38, 39, 43].
Elevation is also used in combination with the other components of PRICE as it decreases the hydrostatic pressure when elevating the injury above the heart, thus decreasing the potential for local edema [43].
NSAIDs have been recommended for inflammation and pain control [38, 41]. Some authors recommend that NSAID use should be restricted during the first 24–72 h due to potential worsening of hemorrhage as a result of anticoagulant effects, but Celecoxib and other COX-2 selective inhibitors might be an option as they do not affect platelets [16, 38]. Other authors recommend limiting the use of NSAIDs to a short-term period during the early stages of healing as they decrease the inflammatory reaction without affecting the healing process or myofiber regeneration [43]. The same study recommends short-term use because long-term use might have an effect on the regeneration of skeletal muscle . Acetaminophen and narcotics may be used as well to help control pain [16, 44]. Alternatively, the use of topical NSAIDs might reduce pain with less risk of gastrointestinal complications.
The use of corticosteroids has been controversial in musculotendinous lesions. Glucocorticoids have led to retardation of tissue regeneration as well as delayed elimination of hematomas and necrotic tissue [43, 45]. Based on the literature, use of glucocorticoids appears to lead to an increase in the strength of affected and non-affected muscles during the initial 2 days of healing versus control groups. However, after 1 week of treatment, injured and non-injured muscles showed weakness when compared to control groups. Alternately, muscular injuries treated with anabolic steroids showed no differences during the first 2 days when compared to controls, but the group treated with anabolic steroids showed an increase in the strength of the injured muscle after 1 week of treatment when compared with the contralateral muscle and with the control group [45].
Therapeutic ultrasound has been widely recommended for the acute treatment of muscular injuries. Typical parameters in application of therapeutic ultrasound for this indication would be a 50 % duty cycle with 1 MHz frequency and 0.7 W intensity, with a duration of between 5 and 15 min. It has been proposed that therapeutic ultrasound, performed within the parameters listed above, could enhance the initial stage of muscular regeneration [43]. However, several studies have found that there is no improvement in healing time when compared with non-treated muscle injuries. Aside from the analgesic effect of the high-frequency ultrasound waves, there is no other additional benefit of ultrasound therapy [43, 46].
Manual therapy techniques are often employed in the rehabilitation of soft tissue injuries. Although many approaches to manual therapy exist, Active Release Techniques® (ART®), Graston Technique®, and Astym® are some of the most popular applications of manual therapy in rehabilitation settings. In ART®, deep digital tension is applied at the affected area, and the patient moves actively through identified sites of soft tissues adhesions from a shortened to a lengthened position. This modality of therapy requires knowledge of the anatomy of the limbs and comprehension of the traversing tissues located at angles. It has been used in cases of tibialis posterior strains with adequate results [38]. Graston Technique®, a form of instrument-assisted tissue mobilization, uses patented stainless steel instruments with beveled edges that are used to manipulate and mobilize the tissue with the goal of decreasing fascia irregularities . The Graston Technique® is based on the belief that treatment in this way will enhance proliferation of extracellular matrix fibroblasts, improve ion transport, and decrease cell matrix adhesions. Astym® also uses a specialized instrument set to mobilize soft tissue by transferring pressure and sheer force to the underlying dysfunctional soft tissue with the goal of increasing fibroblast recruitment and tissue quality.
Experimental studies initially showed that hyperbaric oxygen, or HBO, accelerated the recovery of injured skeletal muscle in animals. However, more recent meta-analysis has shown that HBO might actually increase the sensation of pain in milder degrees of muscular injury [43]. The use of HBO for skeletal muscle injuries is rare and remains experimental.
Although there is plenty of literature indicating the destruction of sarcomeres and muscular damage secondary to eccentric exercises, it has been proposed that a mild eccentric exercise program might protect the muscle from further damage due to adaptation of the sarcomeres, therefore leading to fewer areas of potential instability [47]. The role of eccentric exercise in acute soft tissue injury rehabilitation remains antithetical, and additional investigation would be needed prior to application in the initial phase of a rehabilitation program.
The goal of the sub-acute and chronic phases of tibialis posterior injuries should be directed toward the modification of training regimens and correcting the mechanics of the lower leg as well as training errors [39]. As the muscle fails progressively, the calcaneus rotates into a valgus angle, the arch collapses, and the forefoot gradually abducts, likely producing an acquired flat foot [40]. A key part of the treatment is assessing the need for adequate footwear with shock-absorbing soles and insoles that might fix easily correctable problems. It is also recommended to change shoes every 250–500 miles [39–41]. The use of lace-up shoes is generally recommended to improve foot stabilization. Some patients might be able to correct issues with off-the-shelf orthotics [38–40].
After the acute phase of rehabilitation, the athlete may begin gradually exercising as tolerated. Runners or triathletes may start swimming and cycling, and tibialis posterior strengthening exercises such as heel-ups with a tennis ball held between the medial malleoli can be initiated (Fig. 11.4) [38]. To avoid complete cessation of activities, a 50 % decrease in the weekly running distance, frequency, and intensity may also be recommended to runners for a period of 2–6 weeks. Running on uneven surfaces should initially be avoided, and a synthetic track or other uniform surface of moderate firmness may provide more shock absorption [39]. Running on uneven surfaces may then be gradually introduced and progressed as tolerated [44].
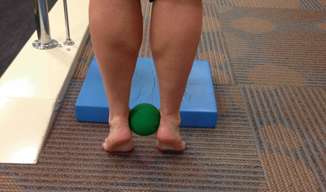

Fig. 11.4
Heel-ups with a tennis ball
The decision to start return to sport-specific practices can be made after two conditions have been met: First, the athlete must be able to stretch the injured muscle as much as the contralateral side; additionally, the athlete must be able to perform pain-free basic movements with the injured muscle. If both conditions have been met, the athlete may start training gradually, preferably under the supervision of an athletic trainer [43].
Flexor Hallucis Longus
Flexor hallucis longus (FHL) injury is the most common lower extremity tendinitis in classical ballet dancers and is sometimes referred to as “dancer’s tendonitis.” This injury is also seen in persons who participate in activities requiring frequent push-off maneuvers and extreme plantar flexion, such as swimmers, ice skaters, long-distance runners, and gymnasts [48, 49]. As flexor hallicus longus injuries are most common in dancers, the rehabilitation techniques in this section are geared primarily toward helping dancers but can be adapted for other individuals.
In dancers, the FHL tendon is generally stressed in the direction of overlengthening as it functions as a medial stabilizer of the ankle in the externally rotated position of the lower limb known as the dancer’s “turnout.” Prevention can be the best form of treatment. FHL injuries can be prevented by reducing turnout of the hip, so the dancer is working directly over the foot. Additional training techniques to help prevent FHL injuries include: avoiding en pointe exercises and hard floors whenever possible, gripping of the floor with the toes when jumping, strengthening the body’s core, and wearing firm, well-fitted shoes. Prehabilitation exercises to improve ankle stability, such as proprioception exercises (wobble board, TheraBand) should be encouraged [49–51].
For post-injury rehabilitation, the focus should be on correction of muscle imbalances, weakness, and faulty technique as mainstays of treatment. For dancers, correction of “over-turning out” should be the first-line treatment, as well as strengthening the anti-pronation muscles of the ankle and the intrinsic muscles of the foot to support the medial longitudinal arch. A careful examination will establish whether there are faults in technique. In conjunction with technique correction, strengthening of the hip and core musculature to support the correct turnout technique is important.
Dancers can relatively unload the flexor hallucis longus tendon during healing by avoiding work on demi-pointe (end-range weight-bearing plantar flexion; Fig. 11.5) and full pointe (Fig. 11.6), and by minimizing jumping due to push-off demands. Optimal floor cushioning would theoretically help to reduce tendon overload, but hard floors in the studio are unfortunately impossible to avoid. In addition, injuries can arise from floors that are too soft or from any rapid change in training surface, so caution should be advised in modifying the training surface. Moreover, dancers are required to wear minimal footwear in the studio (ballet slippers, pointe shoes, etc.), and changing their footwear to rest an injury is next to impossible during participation. However, use of supportive shoes outside of class is a reasonable expectation [52].
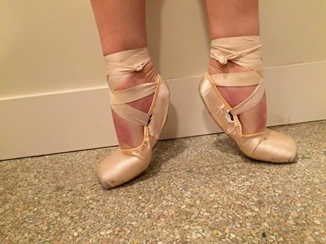


Fig. 11.5
Ballet position—demi-pointe

Fig. 11.6
Ballet position—full pointe
Most authors recommend 6 months of nonsurgical treatment prior to consideration of surgical intervention for flexor hallucis longus injuries. Unfortunately, reported failure rates for nonoperative management range from 40 to 100 %. Common treatment approaches include rest, administration of NSAID medications, ice or contrast baths, gentle stretching, massage, water therapy, and ultrasound [51].
One of the more successful nonoperative treatments is the FHL stretch exercise. To perform this exercise, the patient stands facing the wall with a book placed under the hallux. While keeping the heel on the ground, the ankle is maximally dorsiflexed by flexing the knee. Typically, the stretching is performed for 30 s, repeated three to five times. A more limited protocol of stretching 10 s for ten repetitions with each set of stretches being repeated three to four times daily may be helpful as a general gliding mobilization of the tendon [53].
Stretching, however, is not always an effective treatment because the tissue is often overlengthened and too painful. If stretching aggravates or fails to improve symptoms, immobilization in a walking boot is often necessary. Initially, the walking boot can be used at night for 6 weeks. If symptoms fail to diminish, use of a walking boot (or application of a short leg walking cast extending past the toes) should be applied full time for 6 weeks. If a walking boot is used for immobilization, it can be combined with intermittent use of the FHL stretching exercise as tolerated. After 6 weeks of walking boot use, the patient should progress to a full program of FHL stretching and a formal rehabilitation program as outlined above [49–51].
Intra-tendinous injections of steroids in the FHL, as in all forms of tendinitis, should be avoided because of the risk of weakening or rupturing of the tendon. Ultrasound-guided injection of corticosteroids into the flexor hallucis longus tendon sheath vastly decreases the risk of tendon rupture and has led to clinical success in the experience of the senior author of this chapter. If triggering of the tendon is present, or symptoms are unresponsive to 6 months of conservative treatment, surgical decompression and repair of the tendon may be necessary [51, 53].
Chronic Exertional Compartment Syndrome
Chronic exertional compartment syndrome , or CECS, can occur in any compartment of the extremities, although the leg is the most common location [54].
Conservative treatment is frequently attempted for CECS; however, typically, it has been thought to be generally unsuccessful for athletes who wish to train or compete at a high level. Conservative management generally involves a decrease in activity or load to the affected compartment. This effectively results in detraining of the muscles within the effected compartment and can reduce compartment pressures. After detraining, activity level is gradually increased and titrated based on the patient’s symptoms. During detraining, cross-training exercises like swimming, bicycling, and other low-impact activities are prescribed. Aquatic exercises, such as running in water, are often employed to maintain fitness while improving mobility and strength without unnecessarily loading the affected compartment. Massaging and stretching exercises have also been shown to be occasionally effective in CECS.
In addition to activity modification, biomechanical correction with orthotics and the use of NSAID medications are often prescribed. Pain control with analgesics may be warranted in patients with CECS for acute symptom management, but they play a minimal role in the treatment of this condition. It is generally recommended to avoid compression of the affected limb as this may result in increased compartment pressures. Some athletes have symptoms that are worse on certain surfaces (concrete versus running track or artificial turf versus grass) and symptoms may be relieved by switching surfaces [55].
It should be noted that the presence of muscle weakness in CECS does not necessarily indicate the need for a muscle strengthening program. Exercise brings on the symptoms of CECS and leads to hypertrophy of the muscles within the compartment; therefore, muscle strengthening is problematic for these patients. Only patients with very mild CECS may be able to build strength without precipitating abnormal compartment pressures [56].
Research has suggested that treatment with anti-inflammatory medications, stretching, prolonged rest, ultrasound, electrical stimulation, orthotics, and massage have resulted in limited success, but assessing and correcting biomechanical faults is a core component of rehabilitation for CECS . For example, a hindfoot strike gait pattern leads to increase ground-reaction forces, stride length, and ground-contact time. Thus, it is possible that training and adopting a forefoot running technique may be effective in reducing the symptoms of athletes with CECS who hindfoot strike by adopting a forefoot running technique [57].
Despite the limitations described above, a trial of conservative treatment should be undertaken for most patients with CECS. Along with activity modification, this should include gait analysis, biomechanical correction, and a trial of gait retraining. If conservative therapy is unsuccessful, the patient should be referred for compartment pressure testing and surgical consultation for consideration of release of the effected compartments [55].
Achilles Tendon
Achilles tendon , the largest and strongest tendon in the body, connects the calcaneus to the gastrocnemius and soleus muscles. Due to its function and limited blood supply, the tendon is vulnerable to injury that is difficult to heal. Common pathologies of the Achilles tendon include tendinitis, peritendonitis, tendinosis, and rupture. These conditions are generally caused by overuse and can occur in adolescents and adults.
Cryotherapy is regarded as the single most effective modality for tendon inflammation, with sufficient evidence throughout the literature. An Achilles tendon injury , like any other injury to the body, initiates a cascade of normal inflammatory physiology. During an injury, vasodilatation leads to capillary permeability with fluid extravasation and swelling, metabolic changes, and increased pro-inflammatory cytokines. Cryotherapy combined with compression therapy has been shown to decrease microcirculation at the mid-portion of the Achilles tendon space, promoting preservation of deep tendon oxygen saturation and facilitated venous capillary outflow [58].
Another therapeutic modality, phonophoresis, harnesses the acoustic effects of ultrasound to assist in driving medications into tissue. Acoustic stimuli produce four distinct effects on tissue: thermal, non-thermal, neural, and connected effects. Thermal effects coincide with the absorption of sound energy, and the corresponding heat can be augmented by the increase in frequency; 90 kHz of ultrasound can penetrate tissue to a depth of 10 cm. Non-thermal effects are due to acoustic pressure changes within the tissue, which can be used to help propel medications into soft tissue. Ultrasound also stimulates increased fibroblast activity and increases dispensability of tissues [59].
Iontophoresis is another therapeutic modality for the treatment of Achilles tendinopathy. This modality uses electric stimulation to produce an electric field between two electrodes and uses ions from medication to diffuse through tissue. Iontophoresis is contraindicated for individuals who have a pacemaker, are pregnant, are diabetic, have electrical hypersensitivity, or have skin allergies. Although the patient population was small, a double-blind study reported positive results when iontophoresis was used with dexamethasone as compared to iontophoresis with saline solution. Following treatment with iontophoresis, both groups participated in a rehabilitation program in which 10 participants were followed up at 3, 6, and 12 months. Although both groups noted improvement of Achilles pain during the length of the study, the group with iontophoresis and dexamethasone reported less pain while walking at 3, 6, and 12 months [60].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








