UPPER EXREMITY INJURIES
Acromioclavicular joint (ACJ) sprains are common among young adults who engage in sporting activities, and usually result from falling directly on the acromion or onto an outstretched hand. The forces involved in such a fall drive the acromion inferiorly and superiorly, respectively.
The ACJ is a synovial joint located between the distal clavicle and the acromion. The posterolaterally facing medial facet of the distal clavicle articulates with the anteromedially facing acromion. Static stabilizers of the ACJ are the acromioclavicular (AC) and coracoclavicular (CC) ligaments. The superior, inferior, anterior, and posterior AC ligaments become part of the AC joint capsule. The CC ligaments, trapezoid and conoid, extend from the coracoid process to the inferior surface of the clavicle. The trapezoid is lateral and has a wide insertion on the clavicle, whereas the conoid is medial and has a thin insertion on the posterior tubercle of the clavicle. Dynamic stabilizers of the ACJ are the deltoid and trapezius, also known as the deltotrapezial complex. The deltoid prevents superior and posterior migration of the clavicle. Contraction of the trapezius compresses the ACJ.
Five to 8 degrees of motion at the ACJ facilitates clavicular rotation along the long axis and elevation and retraction of the distal clavicle. This motion aids in maintaining the subacromial space during terminal arm elevation.
ACJ sprain or separation can be diagnosed clinically by localized pain and deformity. The horizontal adduction test compresses the ACJ and causes pain in the presence of injury. The Zanca view radiograph allows for optimal visualization of the joint. ACJ sprains and separations are graded on a scale from I to VI, using the Rockwood classification.
Type I injury results in a sprain of the capsule and AC ligaments, but the AC and CC ligaments remain intact. No clavicular instability can be detected on examination, and radiographs are normal.
Type II injury results in rupture of the capsule and AC ligaments, but the CC ligaments remain intact. The clavicle is unstable under direct stress. Stress radiographic views are negative; however, the lateral clavicle can be slightly elevated.
Type III injury results in complete rupture of the AC and CC ligaments without disruption of the deltotrapezial fascia. On examination, the lateral clavicle appears elevated, with the acromion depressed. The clavicle is unstable vertically and horizontally. ACJ separation is evident on stress radiographs.
Type IV injury results in rupture of the AC and CC ligaments, with posterior displacement of the clavicle into the trapezius. This is evident both clinically and radiographically on axillary view.
Type V injury results in a type III injury with disruption of the deltotrapezial fascia. The lateral clavicle is elevated and the scapula is downwardly displaced. Radiographs demonstrate a 100–300% increase in the clavicle-to-acromion distance (CC distance).
Type VI injury results in inferior displacement of the clavicle into the subacromial or subcoracoid spaces. This usually occurs in the setting of severe trauma and accompanies other injuries.
Patients with type II or III sprains can develop subacromial impingement, rotator cuff pathology, and ACJ osteoarthritis from scapular dyskinesis. If symptoms persist more than 6 months after injury, then corticosteroid injections or surgical repair may be needed.
The acute phase of treatment for type I and II sprains begins at the time of injury and continues through 3 weeks. Therapies include pain-free range of motion (ROM) exercises, soft tissue and scapular mobilization, and isometric strengthening of scapular stabilizers using closed kinetic chain exercises without arm elevation (ie, internal and external rotation exercises at 0 degrees of abduction). Trunk and leg strengthening begins using a wobble board. The patient may progress to arm elevation as tolerated, wear an arm sling for the first 3–10 days, and use analgesics for pain. The recovery phase occurs over the next 3–8 weeks postinjury. Therapies during this period include increasing loads with isotonic closed kinetic chain exercises. The patient may begin active arm elevations. The maintenance phase begins when the patient has full, pain-free ROM and approximately 75% strength compared with the contralateral side. Approximately 12% of young athletes with low-grade sprains undergo surgical repair because of persistent symptoms.
The acute phase of treatment for type III sprains begins with shoulder rest in a sling for 7–14 days. The patient may slowly incorporate active, assisted ROM isometric exercises with internal and external rotation at 0 degrees, shoulder shrugs, and pendulum exercises. After 7–14 days, the patient may remove the sling, beginning the recovery phase of treatment, which includes active ROM isotonic exercises with flexion greater than 160 degrees and abduction to 90 degrees. The maintenance phase begins when the patient has full, painless ROM and approximately 75% strength compared with the contralateral side. Patients with type III sprains may undergo surgical repair if pain or instability persist. Patients with type III separations have alterations in scapular motor planning and scapular dyskinesis that must be addressed through physical and occupational therapy.
Type IV–VI sprains are severe injuries that often occur in combination with other traumatic injuries. For patients with such injuries, noninvasive treatment measures are generally insufficient. Consequently, approximately 75% of young athletes with high-grade sprains undergo CC ligament reconstruction. Most patients, nonsurgical and surgical, are capable of returning to full function with adequate rehabilitation.
Primary external impingement of the shoulder is caused by subacromial or subcoracoid impingement of the rotator cuff. Secondary impingement of the shoulder is caused by abnormal glenohumeral and scapulothoracic motion. Static stabilizers of the shoulder include the glenoid labrum and glenohumeral ligaments. Dynamic stabilizers of the shoulder include the rotator cuff muscles (supraspinatus, infraspinatus, teres minor, and subscapularis). Typically the rotator cuff tendons run along the longitudinal axis of the muscle; however, there are medial and deep components of the tendons that course transversely. They are known as the rotator cable. The rotator interval is a gap in the rotator cuff through which the long head of the biceps tendon passes.
The undersurface of the acromion can be a cause of impingement. Grade I acromion impingement is flat, grade II is concave, and grade III is hooked.
The deltoid and supraspinatus muscles provide superior translational forces to the humeral head, while the infraspinatus, teres minor, and subscapularis provide inferior translational forces. Normally, these opposing forces are balanced. Supraspinatus impingement syndrome occurs when there are unopposed superior translational forces of the humeral head in 30–60 degrees of shoulder abduction.
Partial-thickness tears of the rotator cuff tendons decrease the overall power of the shoulder musculature. This leads to superior migration of the humerus under the acromion, which then further impinges the rotator cuff. The impingement typically occurs in the tendon’s hypovascular zone, which is located 2–6 cm proximal to the insertion, and ultimately leads to full-thickness tears.
In younger patients, supraspinatus tendon degeneration may be induced by repetitive overhead activity, which causes tendon thickening and erosion under the coracoacromial ligament. Secondary impingement can occur in association with humeral instability caused by microtrauma to the static stabilizers of the glenohumeral joint. The weakness of the static stabilizers leads to increased demand on the dynamic stabilizers, causing muscle fatigue. Subluxation of the humeral head occurs anteriorly and superiorly, with impingement on the coracoacromial arch. Upward and anterior scapular tilting can cause increased subacromial contact with the greater tuberosity.
The clinical examination finding most often used to diagnose impingement is resisted initial shoulder abduction causing pain in the deltoid muscle. A positive finding (ie, pain or resisted motion) in any of the following maneuvers is also indicative of impingement.
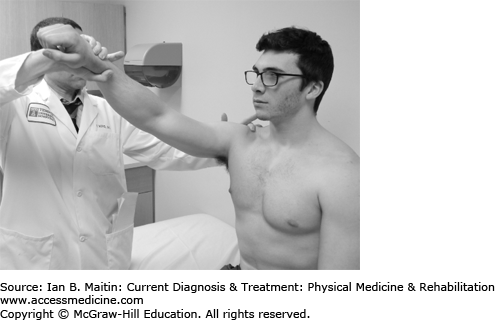
The examiner passively induces 160 degrees of shoulder flexion while stabilizing the scapula (Figure 30-1).
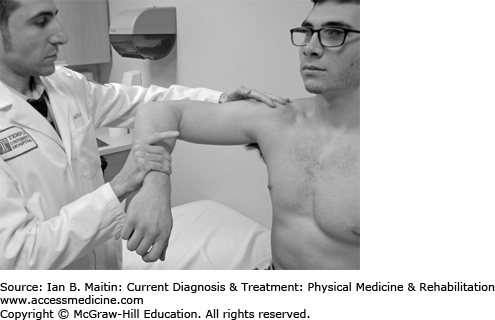
The examiner passively induces 90 degrees of shoulder flexion with maximal internal rotation (Figure 30-2).
The examiner resists shoulder abduction with the patient’s shoulder flexed to 90 degrees in the plane of the scapula with the forearms maximally pronated.
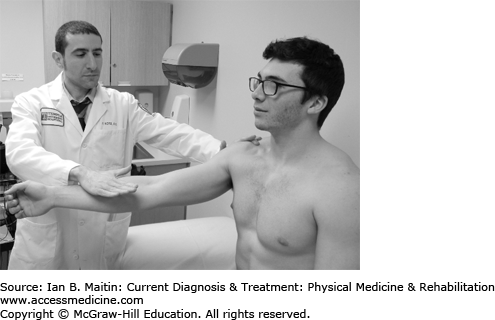
With the patient’s shoulder flexed to 90 degrees, the examiner resists shoulder flexion while the patient’s elbow is extended and forearm is supinated (Figure 30-3).
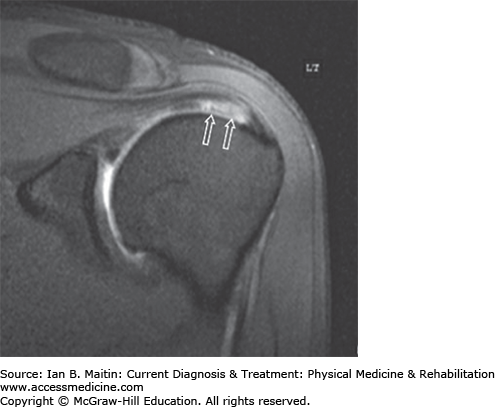
Anteroposterior and outlet-view radiographs are useful in diagnosis. The height of the subacromial space can range from 10 to 15 mm; narrowing of the space to 6–7 mm may indicate impingement. Cross-sectional views using magnetic resonance imaging (MRI) with arthrography and ultrasonography can aid in diagnosis. MRI demonstrates both bony and soft tissue abnormalities. Magnetic resonance arthrography is the gold standard in diagnosing full-thickness tears.
Partial-thickness tears should be graded by the percentage of tendon thickness involved (grade I, < 25%; grade II, 25–50%; grade III, 50–75%). Muscle atrophy with fatty infiltration can be seen on computed tomography (CT) scans (Figure 30–4). The diagnosis of supraspinatus tendinosis secondary to subacromial impingement and bicipital tendinosis can be confirmed using ultrasonography. (See the discussion of musculoskeletal ultrasound in Chapter 39 for more details.) Up to 34% of patients with full-thickness tears are asymptomatic.
The differential diagnosis of external shoulder impingement includes osteoarthritis of the acromioclavicular joint and subacromial subdeltoid bursitis.
Treatment includes nonsteroidal antiinflammatory drugs (NSAIDs) and physical therapy. The latter focuses on strengthening the inferior rotator cuff muscles, excluding the deltoid, which as previously noted is thought to cause the upward humeral subluxation leading to subacromial impingement. Subacromial subdeltoid steroid injection may be beneficial in relieving pain in order to allow greater gains with therapy; however, a significant rotator cuff tear should first be excluded, as an intratendinous injection can cause tendon rupture. Full-thickness tears may require surgical repair.
Internal impingement of the shoulder occurs when soft tissues, such as the joint capsule and labrum, become impinged between the surface articulations of the humerus and the glenoid. Repetitive shoulder abduction and external rotation leads to posterosuperior impingement of the labrum and undersurface of the rotator cuff. This can manifest as labral fraying and partial tears of the rotator cuff. Excessive external rotation loosens the inferior glenohumeral ligament, promoting anterior translation and posterosuperior impingement. Glenohumeral rotation deficit (GIRD) may also be the cause of internal impingement. Repetitive throwing causes a posteroinferior joint capsule contracture, which creates “pseudolaxity” with increased external rotation and decreased internal rotation. This imbalance predisposes the individual to a lesion of the superior glenoid labrum, also known as a superior labrum anterior–posterior (SLAP) lesion. SLAP lesions are most often reported in throwing athletes, and are described in detail in Chapter 29.
The throwing athlete with internal impingement of the shoulder typically reports posterior shoulder pain in the late-cocking phase of pitching, or when the arm is abducted and externally rotated. The affected arm may feel weak and unstable after throwing. Signs of external impingement are usually negative (eg, Hawkins and Neer signs, described earlier). The following radiographic views are used to detect internal impingement: anterior–posterior views (in internal and external rotation), West point, Stryker notch, axillary, and scapular Y views. Common radiographic signs of internal impingement are Bennett lesions (exostosis or calcification in the region of the posterior–inferior glenoid rim), sclerotic changes in the greater tuberosity, an osteochondral lesion or cystic changes in the posterior humeral head, and rounding or remodeling of the posterior glenoid rim. MRI remains the gold standard as the aforementioned radiographic findings are often difficult to detect. Conservative treatment includes shoulder rest, antiinflammatory medications, and cryotherapy. Rest should continue for 4–6 weeks, or until the pain has resolved. Surgical treatment ranges from debridement of the frayed labrum or rotator cuff to complete repair of rotator cuff tendons having 50–75% tears.
Most patients are able to meet goals for recovery after 3 months of physical therapy, and return to work or sport within 6 months.
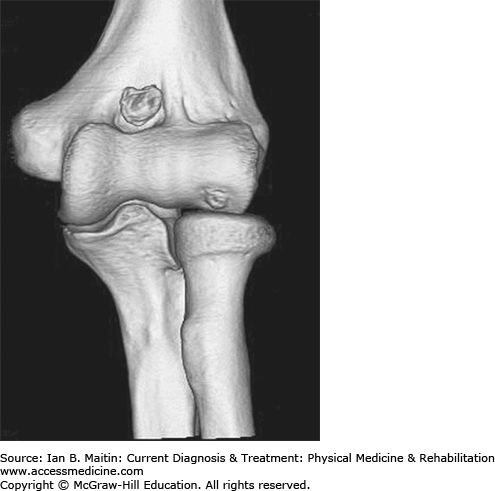
The elbow is the most commonly dislocated joint in the pediatric population and is second to the shoulder in the adult population. Elbow dislocation is more common in males than females and is usually associated with sports such as gymnastics, wrestling, basketball, and football. The most common mechanism is a fall on an outstretched hand, causing axial compression, external rotation of the forearm, or supination, and a valgus moment.
O’Driscoll described a “ring of instability” involving progressive disruption of the elbow, differentiating three stages of disruption. In stage 1, the lateral portion of the ulnar collateral ligament is disrupted, causing posterolateral rotatory subluxation with spontaneous reduction. Stage 2 occurs with continued force, causing incomplete subluxation that leaves the coronoid perched on the trochlea. Stage 3A includes complete disruption of the soft tissues and the posterior part of the medial collateral ligament. Stage 3B includes complete disruption of the medial collateral ligament, causing varus, valgus, and rotatory instability.
Elbow dislocations can likewise be classified into three categories: posterior, divergent, and anterior. Posterior elbow dislocations are subdivided into posterolateral, posteromedial, and pure lateral dislocations. Divergent dislocations are associated with high-impact trauma and radioulnar displacement with damage to the interosseous membrane. Anterior dislocations are rare and are seen in younger patients.
Patients with an acute elbow dislocation present with severe pain and a deformed upper limb. There is usually a history of trauma to the arm. Before attempting reduction maneuvers, a neurovascular assessment should be performed. The wrist and shoulder should also be examined for coinciding injuries, and anteroposterior and lateral radiographs obtained.
Elbow dislocations may be associated with radial head and neck fractures, avulsion fractures of the medial and lateral epicondyles, and coronoid fractures. Neurovascular complications include brachial artery injuries, median nerve entrapment, and compartment syndrome. The median nerve may be displaced posteriorly and become entrapped by an avulsion fracture of the medial epicondyle. The tension placed on the median nerve may “notch” the epicondylar flare of the humerus, producing a Matev sign on radiographs (sclerotic edges of a bony tunnel at the medial epicondylar ridge where the median nerve has been entrapped).
Rapid closed reduction is preferred before soft tissue swelling occurs. Conscious sedation may be necessary to allow muscle relaxation. Closed reduction is performed in prone traction with the elbow extended, using the physician’s thumb to guide the coronoid around the trochlear. Irreducible elbow dislocations may be caused by radial head entrapment within the soft tissues. Postreduction, the elbow should be evaluated for instability. Posterolateral rotatory instability is best assessed using the pivot–shift test, which is positive when a “clunk” is heard or felt as the radius and ulna reduce on the humerus. Radiographs should be obtained postreduction. Widening of the joint space may indicate entrapped intraarticular osteochondral fragments. Surgical intervention is required for unstable reductions, fractures, compartment syndrome, and open dislocations.
Rehabilitation with early, supervised ROM exercise has been shown to be most beneficial. Static flexion and extension splints may be used to aid patients in regaining ROM. Patients with “perched” dislocations have less severe injuries and can expect a full and rapid recovery. Those with more severe injuries may not recover complete ROM of the elbow, lacking full extension. With inadequate bone alignment there is an increased risk for arthritis of the elbow joint.
Osteochondritis dissecans of the capitellum is most commonly seen in teenage boys who participate in sports activities that involve repetitive overhand throwing. Contributing factors may be repetitive valgus stress to the elbow, as in throwing, and immature articular cartilage over the capitellum. These cause local injury to subchondral bone and can lead to avascular necrosis and subchondral osseous changes affecting the humeral capitellum of the dominant hand.
The typical presentation is a 10–15-year old male pitcher with lateral elbow pain and swelling. Patients with stable osteochondritis dissecans present with normal elbow ROM. Radiographs reveal flattening of the capitellum with articular defects; loose bodies may also be present. Three radiographic stages are differentiated. In stage 1, a cystic shadow is visible at the capitellum. In stage 2, the lesion and subchondral bone separate. In stage 3, loose bodies are present (Figure 30–5). The gold standard for diagnosis is MRI, which can aid in staging. CT scans are helpful in detecting loose bodies.
Osteochondritis dissecans of the capitellum should be distinguished from osteochondrosis of the capitellum, which is characterized by capitellar epiphyseal necrosis, regeneration, and calcification in children from 7 to 12 years of age.
Stable lesions are treated with avoidance of repetitive stress, muscle strengthening, and occasionally splinting. Unstable lesions require surgical intervention, including debridement, removal of loose bodies, and, potentially, internal fixation of larger fragments or autologous chondrocyte graft transplantation. Approximately 90% of stage 1 lesions and 52% of stage 2 lesions heal with conservative management consisting of avoidance of heavy elbow use on the affected side for 6 months.
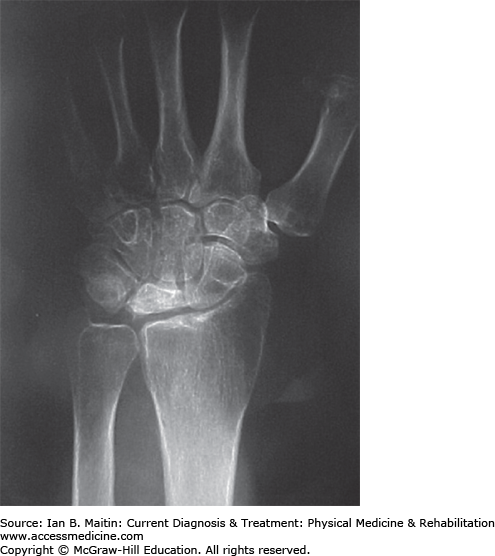
The lunate is the central bone in the proximal carpal row, articulating with the radius, triangular fibrocartilage complex, hamate, and capitate. Multiple etiologic factors can contribute to osteonecrosis of the lunate, also known as Kienböck disease. Mechanical extrinsic factors include a short or long ulna, causing uneven distribution of axial forces; altered radial head inclination; and repetitive trauma. Mechanical intrinsic factors may be related to variations in the shape of the lunate. Vascular factors include interruption of the dorsal and palmar arterial supply; decreased venous outflow; and transient synovitis, compromising vascular supply. Sickle cell disease and thrombocytosis may also compromise vascular supply. Low-grade osteomyelitis has also been linked with this condition.
Osteonecrosis of the lunate is commonly seen in men between 20 and 40 years of age. Patients present with pain and weakness of the affected wrist, but without a history of trauma. Findings are usually unilateral and exacerbated by wrist extension and axial loading. Physical examination reveals dorsal wrist swelling and tenderness over the lunate. Although forearm pronation and supination is preserved, wrist flexion and extension is limited. Four stages of disease are differentiated: stage 1 is similar to wrist sprain, with minimal MRI abnormalities; stage 2 is characterized by lunate hyperdensity that is visible on MRI; stage 3A, by lunate collapse with carpal stability; stage 3B, by lunate collapse with carpal instability; and stage 4, by disruption of the surrounding bones (Figure 30–6).
Treatment varies according to the stage of the disease. Patients with stage 1 disease are generally treated with cast immobilization for 3 months. Those with stage 2 or 3A disease, but ulnar-negative variance, benefit from radial shortening, ulnar lengthening, and capitate shortening. Stage 2 or 3A ulnar-positive variance requires more complex surgical procedures, including vascularized bone graft and external fixation, radial wedge osteotomy, and capitate shortening. In stage 3B disease (lunate collapse with carpal instability), scaphocapitate or scaphotrapezium–trapezoid fusion or proximal row carpectomy are required. Treatment options for patients with stage 4 disease include wrist arthrodesis, arthroplasty, proximal row carpectomy, and wrist denervation. Most patients experience pain relief and increased ROM with revascularization; however, nearly one quarter show disease progression.
LOWER EXTREMITY INJURIES
Osteoarthritis of the hip is the most common pathologic condition of the hip. It is a common complaint in older adults but may also affect younger patients. The prevalence is about 3.1% in the general population. Symptoms are bilateral in about 42% of patients, and genetics appear to play a role in disease progression.
Osteoarthritis occurs when cartilage breakdown leads to release of proinflammatory cytokines, matrix metalloproteinase, and prostaglandins. The inflammatory response promotes subchondral bone remodeling, resorption, neovascularization of synovial fluid, and calcification of the joint cartilage. Over time, these processes lead to the formation of osteophytes, joint space narrowing, and bone sclerosis. Risk factors for premature development of hip osteoarthritis include abnormal hip joint architecture (eg, of the femoral head or acetabulum) and alignment.
Hip pain is usually associated with decreased ROM, made worse with activity, and relieved with rest. Patients with hip osteoarthritis walk with an antalgic gait, characterized as decreased single-limb stance on the painful limb, with short stride length on the contralateral limb. ROM should be evaluated in all planes, including flexion, extension, adduction, abduction, and internal and external rotation. Examination maneuvers should include the FABER test (performed by hip flexion, abduction, and external rotation); a positive test produces groin pain suggestive of intraarticular hip pain, such as hip osteoarthritis.
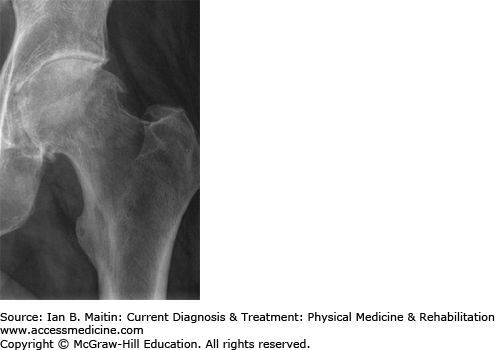
Plain radiographs are usually the first diagnostic imaging study ordered (Figure 30–7). The shortest distance on the radiograph between the femoral head margin and the acetabulum can be used as a measure of the severity of hip osteoarthritis. This is referred to as the minimal joint space (MJS) and is measured medially, laterally, superiorly, and axially. A study by Chu and colleagues found a weak association between joint space width and symptoms.
The Kellgren–Lawrence Grading Scale (KLGS) is another system used to grade hip osteoarthritis. The KLGS takes into account the presence of osteophytes, subchondral sclerosis, and cysts in grading osteoarthritis. Grade I is characterized by possible osteophytes; grade II by small osteophytes and possible narrowing of the joint; grade III by multiple, moderately sized osteophytes, definite joint space narrowing, some sclerotic areas, and possible deformation of bone end; and grade IV by multiple large osteophytes, severe joint space narrowing, marked sclerosis, and definite bony end deformity.
MRI is not necessary but can be used to rule out other conditions in the differential diagnosis of hip pain, including avascular necrosis, labral tear, and fracture. There is a poor relationship between radiographic findings and the need for surgical management.
The differential diagnosis of hip osteoarthritis includes the conditions previously stated above, as well as low back pain, lumbar radiculopathy, sacroiliac joint disorder, meralgia paresthetica, and greater trochanteric pain syndrome.
Patients with progressive osteoarthritis develop joint stiffness, chronic joint pain, and compensatory postural changes that can lead to kyphosis, lumbar radiculopathy, and low back pain. Synovitis and fracture about the hip joint are other causes of concern.
Several nonpharmacologic modalities for treatment of hip osteoarthritis are strongly supported in the literature. These include aerobic, aquatic, and resistance exercises and weight loss. An exercise regimen focusing on ROM, stretching, strengthening, and aerobic conditioning is recommended. Water-based exercises for hip osteoarthritis can decrease pain and increase function in the short term to up to 1 year. Many patients with hip osteoarthritis tolerate cycling and aquatic activities better than high-impact exercises such as stair climbing. However, even with the use of the preceding therapies, a Cochrane review found only a small treatment effect of exercise on hip osteoarthritis pain.
Weight loss is important in the treatment of hip osteoarthritis because body weight is an independent risk factor for the condition. Three to five times the body weight is exerted on the hip while at rest. This increases to eightfold with jogging.
Modalities such as thermotherapy and cryotherapy can be utilized. Additionally, the use of a cane on the contralateral hand will decrease the amount of the force on the arthritic hip. A shoe lift is helpful in correcting leg-length discrepancy.
Pharmacologic modalities include acetaminophen, NSAIDs, tramadol, and intraarticular corticosteroid injections. Acetaminophen is the initial medication choice in the treatment for hip osteoarthritis (maximum daily dose, 4 g). If the patient’s symptoms are not relieved with acetaminophen, NSAIDs should be considered.
Fluoroscopically guided intraarticular cortisone hip injection has been shown to decrease pain and improve function. Limited studies have shown the use of hyaluronic acid to be superior to placebo; however, there is no evidence supporting hyaluronic acid injection as more effective than corticosteroid or other conservative therapies in the treatment of hip osteoarthritis.
Surgical management of end-stage osteoarthritis of the hip includes a total hip replacement with implantation of a prosthetic femoral head and acetabulum. While surgical replacement is usually well tolerated, complications can include infection, prosthetic loosening, and dislocation. Intense rehabilitation is required postoperatively to regain function and strength. (See Chapter 33 for further discussion.)
Femoral acetabular impingement (FAI) occurs when there is noncongruent articulation of the acetabular rim, femoral head, and neck junction, or both. Osseous deformity leads to degenerative arthritis and injuries to the acetabular labrum and cartilage. FAI is differentiated according to the mechanism of deformity into cam-type, pincer-type, or a combination of both. The cam-type (from the Dutch word cam, meaning “cog”) results in a nonspherical femoral head; in the pincer-type, there is overcoverage of the femoral head by the anterior acetabulum.
FAI is usually caused by repetitive microtrauma. The cam-type deformity results in an abnormal head and neck junction, with increased waist radius. The anterior femoral neck loses its concavity and develops a “bump” that impinges on the anterosuperior labrum. In pincer-type FAI, excessive acetabular coverage extends from the anterior acetabular rim prominence over the posterior rim. This overcoverage causes the anterior wall to be displaced in a more lateral direction compared with the posterior wall.
Pain associated with FAI is usually insidious and becomes more severe with time. It is described as hip stiffness, decreased ROM, or pain with weight-bearing activities. It is often associated with groin pain, but lateral thigh pain has also been described. Associated symptoms include clicking, catching, and locking. The pain is worsened with prolonged sitting, stair climbing, putting on shoes, and getting out of a car. On examination, patients have decreased ROM compared with the contralateral limb or pain with the hip in flexion and internal rotation. The classic maneuver for evaluating FAI is the FADDIR test, in which the hip is placed in flexion, adduction, and internal rotation.
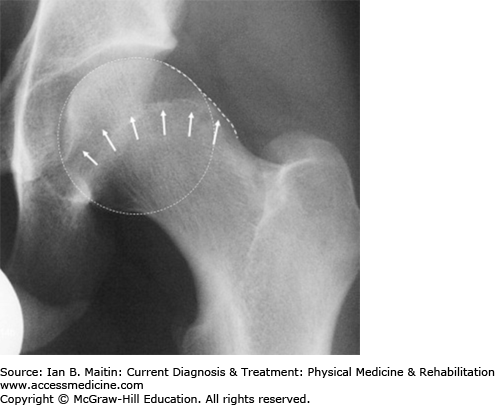
Plain radiographs, especially in anteroposterior and true lateral views, typically show the cross-over sign, an abnormal finding that is characteristic of FAI (Figure 30–8). Measurement of the α angle can help refine the diagnosis. The α angle is the angle that exists between lines drawn from the neck–shaft axis and head center to the aspheric point of the femoral head. An angle greater than 55 degrees is indicative of a cam lesion.
MRI can be performed to evaluate the labrum and articular cartilage. Magnetic resonance arthrography is the most accurate study for diagnosing labral tears and osseous abnormalities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree