Rehabilitation after stroke
Maureen Romanow Pascal and Susan Barker
Introduction
A cerebrovascular accident (CVA), commonly referred to as a stroke, is the interruption of blood flow to brain tissue. The brain tissue that has been deprived of oxygen is damaged or dies. According to the World Health Organization (WHO), the standard definition of stroke is ‘A focal (or at times global) neurological impairment of sudden onset, and lasting more than 24 hours (or leading to death), and of presumed vascular origin’ (WHO, 2006). Strokes can be ischemic or hemorrhagic. Ischemic stroke is the most common type, accounting for 88% of CVAs. Ischemic strokes can be thrombotic or embolic. Thrombotic CVA is caused by a thrombus that develops in an artery supplying part of the brain. Embolic CVA is caused by blood clots that form outside the brain and travel through the bloodstream to the brain (WHO, 2006). Hemorrhagic CVA is the result of arterial bleeding into brain tissue or near the surface of the brain. It is usually the result of trauma, vascular abnormality, or hypertension. Hemorrhagic CVA can be either intracerebral or subarachnoid. Intracerebral hemorrhage is the result of bleeding into brain tissue. Subarachnoid hemorrhage is the result of bleeding into the space between the arachnoid layer and the pia mater.
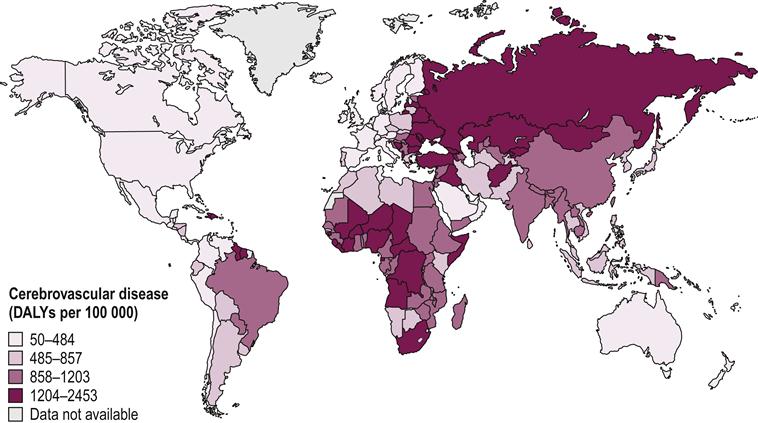
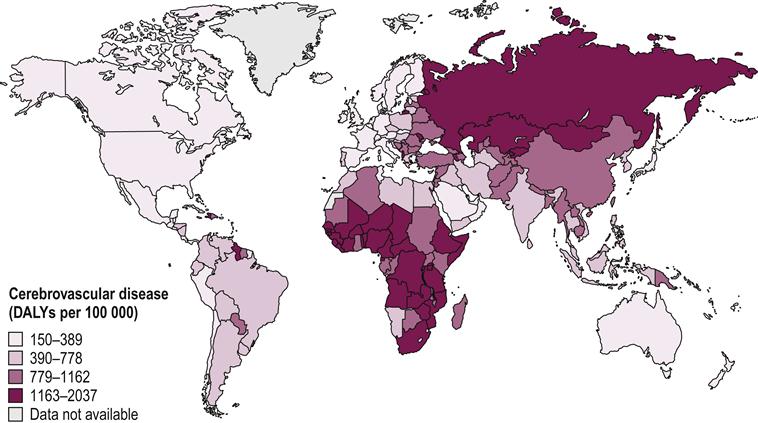
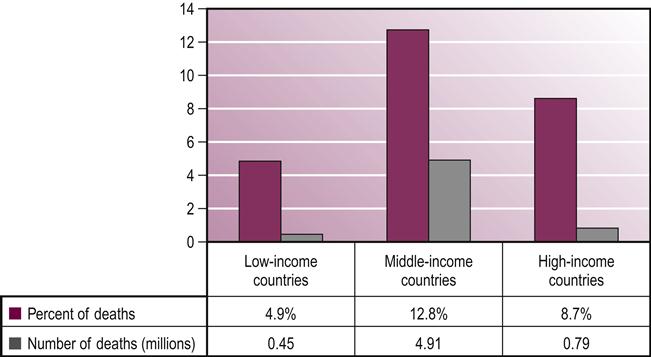
Worldwide, stroke is the leading cause of disability in developed countries. It is estimated there are approximately 12.6 million individuals living with stroke throughout the world. Of these, about 8.9 million live in low- to middle-income countries (Hachinski et al., 2010). Countries in Africa and Northern Asia have the highest concentrations of people living with disability, or disability-adjusted life years (DALYs), for both men and women (Mendis et al., 2011) (Figs 27.1 and 27.2). Stroke is the second leading cause of death, with more than 85% of these deaths occurring in low- and middle-income countries combined, compared to high-income countries (WHO, 2011) (see Fig. 27.3). While mortality due to stroke is expected to decline in developed countries, the burden of disability is expected to increase. This indicates a increasing need for quality stroke rehabilitation, but more importantly, global efforts to reduce the prevalence of stroke.
Risk factors
Some risk factors for stroke are non-modifiable. These include age, gender, race, family history and history of prior stroke or heart attack. CVA risk doubles for every decade beyond age 55. CVA is more common in men than women, and African-Americans and Hispanics are at greater risk for CVA than Caucasians. A person’s risk of CVA is increased if an immediate family member has had a CVA (Goodman & Fuller, 2009).
Among the modifiable risk factors for CVA, hypertension is most important, since about 30% of adults in most countries have hypertension (Mackay & Mensah, 2004). Hypertension accounts for up to 54% of all strokes (Lawes et al., 2008). Patients with atrial fibrillation have five times greater risk of stroke, but treatment with anticoagulants can reduce that risk by two-thirds. Physical inactivity increases stroke risk, and even light physical activity can decrease that risk. Other modifiable risk factors include cigarette smoking or other tobacco use, a diet low in fruits and vegetables, heavy alcohol consumption, being overweight or obese, hypercholesterolemia and the combination of smoking and oral contraceptive use (Kwiatkowski et al., 1999; WHO, 2006).
Signs and symptoms
Signs and symptoms of a possible CVA include weakness or altered sensation of the face, arm, and/or leg, headache, vision changes (field cuts, blurriness), confusion, dizziness and alterations in speech (Sullivan et al., 2004; WHO, 2006). Development of these signs or symptoms indicates the individual should receive immediate medical attention. Other symptoms may include headache, dizziness or vertigo, seizure or difficulty swallowing. If these symptoms are combined with weakness or sensory changes, it is likely the individual is having a stroke. If the stroke is ischemic, blood supply may be restored through a thrombolytic agent such as tissue plasminogen activator; however, this treatment has only been demonstrated to improve outcomes if administered within the first 3 hours of the event (Clark et al., 1999; Kwiatkowski et al., 1999).
Diagnosis
According to the World Health Organization’s STEPS Stroke Manual, ‘stroke is a clinical diagnosis, and is not based on radiological findings’. However, in most developed countries, definitive diagnosis of stroke is often made based on results of a computed tomography (CT) or magnetic resonance imaging (MRI) scan. CT is used more commonly than MRI, as CT is generally more available and less expensive than MRI (Calautti & Jean-Claude, 2003). Both CT and MRI can provide information about areas of infarction or hemorrhage (Kidwell et al., 2004). Diffusion-weighed MRI has been shown to be superior in the diagnosis of acute ischemic stroke, which is an important factor in the decision about the use of thrombolytic medication (Etgen et al., 2004). However, since CT can exclude the diagnosis of hemorrhagic stroke, can be performed more quickly than MRI and is more readily available, it remains the more common imaging modality (Edlow, 2011). In addition to CT and MRI, echocardiography and ultrasound may be used to identify the location of the blood clot responsible for an ischemic event (Gunaratne, 2012).
Prognosis
Impaired motor function after a stroke may lead to long-term disability. Early return of motor function is a good prognostic indicator of future functional improvement. Regaining voluntary shoulder and finger movements within 7 days of a stroke is associated with a good return of arm function (Nijland et al., 2010). Return of leg strength within 1 week is a good predictor of return to independent walking (Stinear, 2010). In a study conducted in The Netherlands, Kwakkel et al. found that, at 5 weeks post stroke, physical therapists and occupational therapists are able to accurately predict a patient’s walking ability and manual dexterity at 6 months post stroke (Kwakkel et al., 2000).
Imaging modalities may be used to determine prognosis after stroke. Functional MRI (fMRI) and transcranial magnetic stimulation (TMS) both show potential for more widespread use as prognostic tools. fMRI can be used to evaluate the cortical activation patterns used to perform a functional movement. The pattern used by a patient after a stroke is well-correlated with level of recovery and outcomes (Carey et al., 2002; Ward et al., 2003; Ward & Cohen, 2004; Stinear, 2010). Patients who demonstrate activation maps similar to controls (i.e. they activate the left cortex for right-sided movements) have fewer residual impairments. Patients who demonstrate activation of the primary motor cortex ipsilateral to the lesion, plus bilateral activation of supplementary areas, generally have greater impairments and a poorer outcome (Ward & Cohen, 2004). Due to the limited availability and costs associated with performing fMRI it is not currently in wide use.
TMS is an imaging modality that shows some potential for prognosis in the future. TMS can be used to stimulate the primary motor cortex and elicit a motor response peripherally. The presence or lack of evoked potentials in the targeted muscles provides information about the integrity of the corticomotor pathway. Similarly to fMRI, the test looks at the patient’s ability to use the cortex on the side of the brain most affected by the stroke (ipsilesional) to control the contralateral extremities. As with fMRI, the ability to use the ipsilesional cortex to elicit motor function is associated with improved functional outcomes. Both fMRI and TMS are used for testing upper extremity more than lower extremity function (Stinear, 2010).
Severity of stroke can play a role in prognosis. Patients who sustain a severe middle cerebral artery stroke tend to have a poor prognosis for functional use of the affected upper extremity. The chance of recovery declines if it takes longer for the patient to make functional gains, or to regain active hand motion (Kwakkel et al., 2000).
There are many other factors that will affect a patient’s outcome after stroke, including the presence of comorbidities such as hypertension, diabetes mellitus and overweight and obesity. The presence of cognitive or perceptual limitations may limit understanding of directions, negatively affecting a patient’s motivation or insight into the deficits of the stroke. None of these factors precludes a good outcome, but they may increase the time it takes a patient to demonstrate functional improvement during rehabilitation.
One specific problem that tends to lengthen the time for rehabilitation is pusher syndrome, a disorder related to a stroke that affects the right or left posterolateral thalamus. In pusher syndrome, or contraversive pushing, the patient shifts excessive weight onto the weaker side, resulting in difficulty maintaining upright sitting or standing. The patient has an impaired perception of vertical, does not attempt to correct the faulty posture and resists correction by another individual. Intervention for pusher syndrome includes using a landmark or visual cue to help the patient identify vertical in the environment. Pusher syndrome rarely lasts more than 6 months post stroke, so although rehabilitation takes longer, the long-term outcome tends not to be greatly affected (Karnath & Broetz, 2003).
One comorbidity that can affect the outcome is metabolic syndrome (see Chapter 46). Metabolic syndrome increases the risk of diabetes mellitus, heart disease and stroke. A patient with metabolic syndrome has at least one of the following factors: large waist circumference, high triglycerides, low HDL cholesterol, insulin resistance and/or hypertension (National Heart, Lung, and Blood Institute, 2011). In one recent study by Mi et al., Chinese individuals with metabolic syndrome, and specifically those with central obesity, were found to be at higher risk for having another stroke within a year of the first stroke (Mi et al., 2012). This finding has implications for patient education about healthy lifestyles, both before and after a stroke.
One other factor that greatly affects prognosis is whether or not the patient will be given the opportunity to participate in a quality rehabilitation program. WHO and the World Stroke Organization both recommend rehabilitation services at a center that specializes in the treatment of stroke (WHO, 2006; Hachinski et al., 2010). The interventions that may be used to promote recovery are discussed below.
Intervention
Several impairments and functional limitations may occur after a stroke. Hemiparesis involving the upper or lower extremity, or both, is one of the most common impairments that may need to be addressed by occupational therapists and physical therapists. Patients may also experience sensory loss or altered sensation in the area of the body affected by the stroke. Other common impairments include decreased balance, sensory, visual and perceptual deficits, impaired cognition, impaired communication, decreased coordination, increased tone and spasticity and decreased motor control. Functional limitations often include decreased functional mobility in bed, transfers, gait and activities of daily living (ADLs), especially those that are usually bimanual, e.g. dressing and bathing. The large spectrum of possible impairments in body structure or function, activity limitations and participation restrictions is the reason a team approach that includes physical and occupational therapists, speech-language pathologists, physicians, nurses and other health professions is crucial to the rehabilitation approach.
The paresis following a stroke appears related to several structural and physiological changes that occur after a stroke, including a decrease in the number of muscle fibers, change in the types of muscle fibers and muscle recruitment patterns, and decreases in peripheral nerve conduction velocity. The functional result is a decrease in ability to produce adequate muscle force (Patten et al., 2004).
Improving physical function plays an important role in quality of life after a stroke. Duncan et al. have researched quality of life in people with stroke and have found that decreased physical abilities have the greatest effect on quality of life after stroke. Loss of hand function is reported as the most disabling (Duncan et al., 2003).
One of the most commonly used treatment interventions for post-stroke rehabilitation is the Bobath approach, or neurodevelopmental treatment. This approach focuses on encouraging, or facilitating, normal movement and inhibiting abnormal movement patterns. Strengths of the Bobath approach are that many of the treatment techniques are designed to encourage increased functional mobility, and treatments are often performed in functional positions. Many experienced therapists who use the Bobath method apply motor learning principles and perform techniques during functional activities (Lennon & Ashburn, 2000). Another strength of this approach is that it can be performed with minimal equipment, making it a good choice for therapists who have limited resources for rehabilitation. The Bobath approach has evolved to focus less on the reacquisition of normal movement and more on the use of problem-solving strategies during functional tasks, focusing on encouraging postural control (Huseyinsinoglu et al., 2012). Despite its wide use, there is currently no evidence that the Bobath approach is more effective than other methods used in stroke rehabilitation. Conversely, there is also no evidence that it is an ineffective approach (Paci, 2003). Due to the training involved, and the variety of techniques that may be employed in the Bobath method, researchers have found it difficult to perform controlled studies of this method.
Other common methods used in stroke intervention include proprioceptive neuromuscular facilitation, and intervention approaches developed by Brunnstrom, Rood, Johnstone and Ayres (O’Sullivan & Schmitz, 2007). Like the Bobath method, the effectiveness or ineffectiveness of these strategies has not been supported by controlled research studies (Van Peppen et al., 2004). Similar to the Bobath approach, these interventions can be performed with little equipment.
It is now well acknowledged that stroke rehabilitation must be designed to maximize the neuroplasticity that can occur as the nervous system attempts to recover. One important factor in promoting neuroplasticity and recovery is making the rehabilitation activities salient, specific and functional. The activities need to have enough intensity and repetition to promote learning (Kleim & Jones, 2008). The task-oriented approach is based on the concept that learning is goal-oriented (Gordon, 2000). While the task-oriented approach does not preclude hands-on activity, it does imply that the patient participates in some exploration of the task, including trial-and-error. One specific method based on the task-oriented approach is the Motor Relearning Programme. A Norwegian study demonstrated that this method can be effective in improving motor function and performance in ADLs in patients with acute stroke (Langhammer & Stanghalle, 2000).
Because the paresis that results from stroke is related to functional limitations, strength training is an intervention that may be appropriate in post-stroke rehabilitation (Canning et al., 2004; Morris et al., 2004; Patten et al., 2004; Forrester et al., 2008). Several studies have demonstrated functional improvements in patients who participated in both strength training and task-oriented functional training (Morris et al., 2004; Patten et al., 2006; Jørgensen et al., 2010). There does not seem to be a link between strength training and increased spasticity (Patten et al., 2004).
Modalities that are frequently used in stroke rehabilitation include functional electrical stimulation (FES) and neuromuscular electrical stimulation (NMES). A recent innovation in the use of FES for the lower extremity is a neuroprosthesis, a device worn by the patient in place of an ankle–foot orthosis or hand splint. The device incorporates FES to promote proper timing of stimulation of muscles during a functional activity. In the lower extremity it is designed to stimulate the ankle dorsiflexors during the swing phase of gait to help reduce or eliminate foot drop. Some lower extremity neuroprostheses can also stimulate ankle plantarflexors at the end of stance to improve push-off (Mesci et al., 2009; Embrey et al., 2010). Currently there is limited evidence to support the use of FES to increase lower extremity strength (Ferrante et al., 2008), and NMES to increase upper extremity strength (Van Peppen et al., 2004). There is strong evidence to support the use of NMES to both decrease inferior subluxation of the glenohumeral joint, and to increase shoulder external rotation passive range of motion (Van Peppen et al., 2004).
Some of the newer interventions that have been developed for rehabilitation of patients with stroke target specific impairments or functional limitations. One of these is constrained-induced therapy (CIT), which is also known as constraint-induced movement therapy and forced use. This intervention targets the hemiparetic upper extremity. A constraining device such as a sling or mitt is applied to the stronger upper extremity to promote increased use of the affected arm (Mark & Taub, 2002). The current protocol requires ‘massed practice with the more affected arm on functional activities, shaping tasks in the training exercises, and restraint of the less-affected arm for a target of 90% of waking hours’ (Mark & Taub, 2002). The protocol has been most successful with patients who have some ability to extend the affected wrist and fingers. It has been less successful in patients with little active movement, although improvement is still possible (Mark & Taub, 2002; Lin et al., 2010). Wolf et al. demonstrated CIT can promote recovery of hand function in patients who are 1 year post stroke (Wolf et al., 2006).
An intervention that specifically targets walking ability is body-weight supported treadmill training (BWS-TT). In this intervention, a portion of the patient’s weight is suspended using a sling attached to an overhead harness. The patient is then assisted in performing gait training on a treadmill. Results from several randomized controlled trials indicate that BWS-TT can help to increase endurance for walking and improve gait speed (Hesse, 2004; Peurala et al., 2005; Lindquist et al., 2007; Tilson et al., 2010). Current evidence does not support using this method to improve walking ability or postural control, except with a specialized split-belt treadmill (Van Peppen et al., 2004; Reisman et al., 2007). In contrast, gait training on a treadmill without body weight support has been shown to improve walking ability (McCain & Smith, 2007; Luft et al., 2008).
Some interventions that are currently being studied use advanced technology to help reduce impairments and functional limitations after a stroke. These include the use of robotic training to improve gait and upper extremity motor control (Lum et al., 2002; Stein et al., 2004; Riener et al., 2005; Frick & Alberts, 2006) and virtual reality (Merians et al., 2002; Weiss et al., 2003; Deutsch et al., 2004). Some interventions, such as mirror therapy (Sütbeyaz et al., 2007; Yavuzer et al., 2008) and aquatic therapy (Noh et al., 2008; Chon et al., 2009), require less technology and have recent research supporting their use. As research in these areas continues, they will likely prove to be valuable adjuncts to current physical therapy practice.
Conclusion
Stroke is a global problem that can result in a multitude of impairments and functional limitations. Improvement of healthcare and public awareness of the importance of reducing risk factors may help to decrease the incidence and severity of this condition, and decrease the global burden of this non-communicable disease. There are currently many types of physical therapy interventions used to improve the functional abilities of patients after a stroke. More randomized, controlled clinical research is needed in this area to help therapists make informed decisions about which interventions are most appropriate for the patients with whom they work.








