Rationale Use of Antimicrobials in Periprosthetic Joint Infection
Joanna L. Cole
John Segreti
Case Presentation
Mr. T is a 50-year-old male with type-2 diabetes mellitus, and osteoarthritis. Due to persistent pain in the left hip that was refractory to conservative therapies, he underwent a left total hip arthroplasty (THA) with placement of porous fiber metal femoral stem and head, with Trilogy acetabulum and Longevity polyethylene liner. He received cefazolin as perioperative surgical prophylaxis. His postoperative course was complicated only by urinary retention following removal of a urinary catheter. Thirteen days following surgery, he developed increased pain in the left hip, a low-grade fever, and presented for evaluation after symptoms persisted for 2 days. Physical examination of the hip was unremarkable. Examination of peripheral blood showed a white blood cell (WBC) count of 11,500 cells/mL, ESR of 73 mm/hr (age-adjusted normal ≤25 mm/hr), and CRP was 374.2 mg/L (normal ≤5.0 mg/L). Synovial fluid was aspirated; the cell count revealed a white blood cell count of 21,763 cells/mL with a predominance of neutrophils (85%). Gram stain of synovial fluid revealed gram-positive cocci in pairs. Aerobic cultures grew a beta-hemolytic streptococcus, identified as Group B streptococcus/Streptococcus agalactiae (GBS). Radiograph of the hip showed a stable prosthesis. He was taken for surgical debridement of an infected prosthesis. Gross purulence was seen in the joint at the time of surgery and the prosthesis was noted to be well fixed. Six samples were obtained for aerobic, anaerobic, fungal, and acid-fast bacilli (AFB) cultures. Periprocedural cefazolin was administered after cultures were obtained. Intraoperative frozen section reported acute inflammation, and final pathology of synovial tissue had >15 neutrophils/hpf. All six surgical specimens grew GBS. The infected tissues were debrided and the polyethylene liner was exchanged. Postoperative day 2, his peripheral WBC count was 11,000 cells/mL and fevers had resolved.
Periprosthetic joint infections (PJIs) are an uncommon, but serious complication of THAs. Infections of hip prostheses occur less commonly than total knee arthroplasties and are estimated to occur in 0.2% to 1.2% of arthroplasties; in the United States, the burden of infection has increased from 1990 to 2004 (1). Infection may result in significant morbidity and functional limitation for the patient and is associated with twice the length of hospitalization and excess cost of nearly $6,000 per incidence (1,2). Risk of infection has been related to several patient risk factors: age, obesity, rheumatoid arthritis, and obesity as well as surgical factors such as revision arthroplasty and operative time (3,4). Arriving at the diagnosis may be simple, such as in the patient presenting acutely with classic symptoms of fever and acute pain in the joint, or challenging, such as the patient presenting with a more indolent course and joint dysfunction. The ultimate goal of therapy for PJI is to eliminate the infection and restore or maintain function in a pain-free joint. For most patients a combination of surgical intervention and antimicrobial therapy will be required. The optimal surgical management will depend on many factors, including the duration of symptoms, virulence of infecting agent, stability and fixation of the prosthesis, adequacy of bone stock, underlying patient comorbidities, and surgeon experience (5). Appropriate antimicrobial therapy also depends on several factors, including pathogen susceptibility, expected antibiotic pharmacodynamics, and side-effect profile. While infection eradication is the preferred outcome, suppression of infection is occasionally necessary when removal of an infected prosthesis is contraindicated because of patient refusal, presence of comorbidities, or expected functional outcomes are unacceptable. This chapter will review the management of PJIs, with focus on pharmacologic therapy.
Diagnostic Considerations
Infection of a hip prosthesis may arise from direct inoculation at the time of surgery, or in the perioperative period by direct extension from the wound, or via hematogenous route in the setting of bacteremia. Classification schemes have been devised that attempt to describe the origin of infection and the likely causative organism (6,7). Classification that is based purely on time elapsed since surgery
may be limited. The timing of clinical manifestations from infection acquired in the perioperative period may differ depending on the virulence of the involved pathogen. Low virulence organisms may manifest in a delayed manner. Conversely, hematogenous seeding may occur at any time, with the responsible pathogen predicted by the nature of bacteremia, with most common sources being skin and soft tissue (46%), dental (15%), and urinary (13%) (8). Tsukayama et al. (9) propose a classification that was found to be useful for descriptive as well as therapeutic purposes: (1) early infection (<28 days from surgery), (2) late chronic infection (>28 days with an insidious course), (3) acute hematogenous infection (acute onset of symptoms in a previously well-functioning joint), and (4) positive intraoperative cultures (2 or more positive cultures for the same organism in joint revision for aseptic loosening).
may be limited. The timing of clinical manifestations from infection acquired in the perioperative period may differ depending on the virulence of the involved pathogen. Low virulence organisms may manifest in a delayed manner. Conversely, hematogenous seeding may occur at any time, with the responsible pathogen predicted by the nature of bacteremia, with most common sources being skin and soft tissue (46%), dental (15%), and urinary (13%) (8). Tsukayama et al. (9) propose a classification that was found to be useful for descriptive as well as therapeutic purposes: (1) early infection (<28 days from surgery), (2) late chronic infection (>28 days with an insidious course), (3) acute hematogenous infection (acute onset of symptoms in a previously well-functioning joint), and (4) positive intraoperative cultures (2 or more positive cultures for the same organism in joint revision for aseptic loosening).
Establishing the diagnosis of PJI most commonly utilizes a combination of synovial fluid analysis, histopathologic examination, intraoperative appearance, and bacterial cultures (10). Preoperative synovial fluid analysis can be an aid in diagnosis of infection and aid surgical planning (11). In review of 220 patients undergoing total hip revision, synovial fluid WBC count and neutrophil predominance were found to be sensitive and specific markers of infection; notably the WBC counts may be much lower in prosthetic joint infection than native joint infection (12,13). Microbiologic diagnosis of the infecting organism is necessary for directing antimicrobial therapy. Preoperative synovial fluid cultures may be positive in up to 80% of patients with PJI (11,14) and can be supportive if same pathogen is isolated at the time of surgery. Inoculating synovial fluid into blood culture media instead of using swabs for agar plating can significantly increase the yield of cultures, particularly for fastidious organisms (15,16). At the time of surgery, multiple tissue samples can improve diagnostic yield; when five or six specimens are taken, two or more positive cultures for the same organism is highly predictive of infection (likelihood ratio 3.3 to 7.6) (17). Tissue samples are preferred over swabs for cultures. Other methods have been developed aimed at improving microbiologic diagnosis. Sonication of the explant has been used to disperse adherent bacteria from the prosthesis itself, and may have better sensitivity compared to periprosthetic tissue cultures, particularly in patients who have previously received antibiotics (18). Molecular techniques that utilize microbe-specific genetic information may be promising in future diagnostic algorithms. Polymerase chain reaction (PCR) or in situ hybridization of bacterial 16S rRNA may improve detection of bacteria when cultures are negative (19,20). Finally, immunofluorescent microscopy, using organism-specific monoclonal antibodies as probes, has been proposed as a rapid adjunct to traditional culture methods (21). However, problems with methodologic standardization, cross contamination for PCR methods, and limited availability in clinical settings are main challenges for broad application of these methods. In addition, they lack capacity to evaluate sensitivity of infecting organism to specific antimicrobial therapy, which is essential for appropriate therapy.
Microbiology and Pathogenesis
The microbiology of PJI is a reflection of both the most common routes of infection, local inoculation and hematogenous spread, as well as pathogenic properties of the infecting organism. Table 98.1 shows the distribution of the most common microbiologic causes of infection in the prosthetic hip (8,9,11,12,13,22,23,24). Gram-positive organisms, especially staphylococcal species, are common colonizers of the human skin and oral cavity and make up the majority of both early and late infections. Among gram-negative bacteria, normal flora of the human gastrointestinal tract, such as Escherichia coli and Enterobacter cloacae are commonly implicated. Pseudomonas aeruginosa found environmentally as well as commensally, remains an important pathogen. Prosthetic hip infections with gram-negative organisms occur more commonly in older patients and patients with older prostheses (25). Anaerobic bacteria, most commonly Peptostreptococcus spp. and Propionibacterium acnes cause PJI less frequently, but are an important consideration as they require specific anaerobic culture media and longer incubation time compared to aerobic bacteria (26). Given the relative difficulty in identifying these organisms in culture, one may need to give consideration to anaerobic involvement in mixed infections, despite negative anaerobic cultures.
There are a myriad of unusual pathogens that have the capacity to cause periprosthetic infections. Epidemiologic exposure and underlying immune status of the host are principal risk factors for many unusual pathogens. Unusual infections are more commonly seen in patients with underlying immunosuppression from disease or disease treatment (e.g., TNF-alpha-blockers). Some agents may be difficult to identify due to the need for specific culture media enrichment, like Abiotrophia sp. (previously nutritionally variant streptococcus), Brucella, and Listeria monocytogenes (27). Fungal infections of prosthetic joints are rare and tend to occur most commonly in patients with underlying risk factors; Candida and Aspergillus are the most commonly implicated fungi, with endemic fungi and molds noted in isolated case reports alone (28,29,30,31,32). Mycobacterium infection is a rare cause of prosthetic joint infection. The “rapid growers,” such as M. abscessus and M. fortuitum, are ubiquitous in the environment and can cause PJI in patients
without obvious risk factors (33). Mycobacterium tuberculosis (MTB) skeletal infection is the second most common manifestation of MTB disease and can be present in prosthetic joint in patients with epidemiologic risk factors, and is more common in patients treated with steroids or immunosuppressants. Infection can be due to delayed local reactivation in a previously affected joint, or due to hematogenous seeding of the prosthesis during pulmonary reactivation with dissemination. Often, previous MTB infection is not known, and diagnosis of PJI may be delayed and potentially misidentified if acid-fast–specific microbiologic studies are not performed (34,35). In up to 26% of cases, despite evidence of purulence, drainage, or the histopathologic diagnosis of infection, cultures fail to identify a pathogen. While fastidious or unusual organisms may occasionally be responsible, prior antibiotic use has been a major risk factor for culture-negative PJI (36,37).
without obvious risk factors (33). Mycobacterium tuberculosis (MTB) skeletal infection is the second most common manifestation of MTB disease and can be present in prosthetic joint in patients with epidemiologic risk factors, and is more common in patients treated with steroids or immunosuppressants. Infection can be due to delayed local reactivation in a previously affected joint, or due to hematogenous seeding of the prosthesis during pulmonary reactivation with dissemination. Often, previous MTB infection is not known, and diagnosis of PJI may be delayed and potentially misidentified if acid-fast–specific microbiologic studies are not performed (34,35). In up to 26% of cases, despite evidence of purulence, drainage, or the histopathologic diagnosis of infection, cultures fail to identify a pathogen. While fastidious or unusual organisms may occasionally be responsible, prior antibiotic use has been a major risk factor for culture-negative PJI (36,37).
Table 98.1 Microbiology of Prosthetic Hip Infections | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
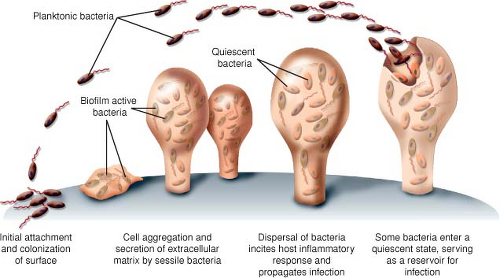
Discussion of the full pathogenesis of prosthetic joint infection is beyond the scope of this chapter, but understanding the means with which bacteria form infections on prosthetic surfaces is helpful in understanding approaches to management. Prosthetic joints as foreign surface may promote infection by decreasing the potency of host neutrophils, decreasing the inoculums required for infection, and providing surface area for biofilm formation (38,39,40). Biofilms form on inert or living surfaces when bacteria adhere irreversibly to the surface, form microcolonies, and produce an extracellular matrix. The major pathogens of prosthetic joint infections, such as staphylococcal spp. and Pseudomonas, are rich in biochemical properties that allow rapid adhesion to surfaces and formation of an extracellular glycocalyx composed of carbohydrate moieties of proteins that protrude from the free surface of the plasma membrane. Figure 98.1 graphically demonstrates the process of biofilm formation. Organisms may progress from sessile forms, adherent to the surface, to free-floating planktonic forms that disperse and then be responsible for the clinically overt manifestation of infection. In addition, organisms within the biofilm enter a slow-growing state that may serve as a reservoir for continued infection if the device is not removed (41). Biofilms present a challenge for pharmacologic therapy as this growth confers increased resistance to antimicrobial therapy through mechanisms reliant on the multicellular nature of biofilms distinct from the typical modes of bacterial resistance (40). The extracellular matrix may prevent diffusion of antibiotics to the site of bacteria; the altered rate of growth likely limits many antibiotics whose mechanism of action is dependent on cellular division, like beta-lactams; and differences in the local chemistry within the biofilm may change the properties of antibiotics, for example, localized areas of anaerobic or acidic conditions may reduce effectiveness of aminoglycoside antimicrobials (39,41,42,43,44). Biofilm formation may also explain the tendency for some prosthetic joint infections to occur in delayed or subacute fashion, as well as the observation that antibiotics may improve clinical symptoms but is then followed by recurrence shortly after their cessation.
General Management Principles
The goals of therapy for prosthetic joint infections aim to cure the infection, prevent recurrence, maintain or restore pain-free joint function, and reduce mortality. Surgical intervention is required to control infection and remove diseased tissue. Options for surgical management include debridement with modular femoral head and polyethylene liner exchange with retention of other well-fixed components, debridement with complete implant removal and revision with a new implant in a single-stage revision arthroplasty, a two-stage replacement arthroplasty—the initial stage including debridement and implant removal, interim antibiotic therapy and revision at a second stage, debridement and resection arthroplasty and no reimplantation, and hip
disarticulation. The factors involved in choosing appropriate surgery for each patient will be addressed in greater detail elsewhere in this textbook.
disarticulation. The factors involved in choosing appropriate surgery for each patient will be addressed in greater detail elsewhere in this textbook.
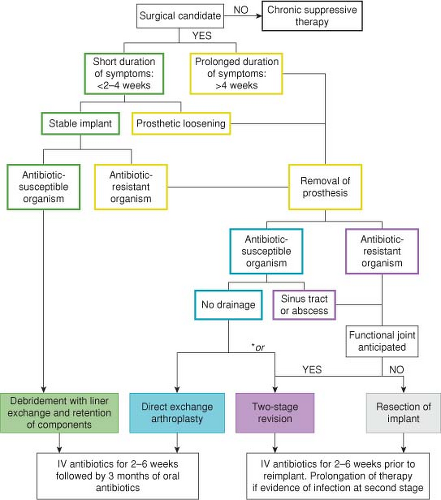 Figure 98.2. Algorithm for management of prosthetic joint infection of the hip. *Direct exchange arthroplasty or two-stage revision may be chosen at surgeon’s discretion. |
Antimicrobial therapy accompanies surgical management strategy, and an algorithmic description is presented in Figure 98.2. In brief, debridement with retention of components is successful in 60% to 90% of reported cases of patients with early infections (45,46,47,48,49). Higher failure rates are associated with S. aureus infections, sinus tract formation, and duration of symptoms of more than 2 weeks prior to debridement. Direct-exchange arthroplasty (debridement, implant removal, and new implant reimplantation in one stage) is commonly employed in cases of early PJI, and is more successful with monomicrobial infections with low virulence organisms that are highly susceptible to standard antibiotics, prolonged antimicrobial therapy, and complete debridement of involved tissues (50). Two-stage reimplantation with interval antibiotic therapy has been associated with rates of success over 90% (51,52,53,54), but can be associated with limited mobility in the interim period as well as the added morbidity of a second surgery. In patients with chronic symptoms, draining sinus tracts, or infections with difficult to treat organisms, the two-stage procedure is recommended. Despite several weeks of antibiotics, reinfection after second-stage revision occasionally occurs. Delay of revision, providing an antibiotic-free interval, may improve detection of patients who have persistent infection by allowing clinical symptoms to recur, as well as improving the yield of cultures at the time of revision. Positive histopathology at the time of revision correlates with culture positivity, though sensitivity of frozen section is only 25% to 28% and thus negative histology may not reliably rule out infection (55). Unfortunately direct comparison of two-staged revision arthroplasty and single-surgical management is not possible as published data have significant heterogeneity among study design, surgical management, and patient characteristics (51,56).
Finally, there are a number of scenarios in which adequate surgical management is not possible, or persistent infection is diagnosed only after second-stage revision has been completed, and chronic suppressive therapy of the infection is required. Suppressive therapy must be considered in patients with contraindications to surgery, with a well-fixed prosthesis that would be too difficult to remove or would leave
an unreconstructable situation, with poor bone stock that would result in limited function of the joint, or merely based on patient choice. Table 98.2



an unreconstructable situation, with poor bone stock that would result in limited function of the joint, or merely based on patient choice. Table 98.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree