Pseudogout (Calcium Pyrophosphate Dihydrate Crystal Arthropathy)
Theodore R. Fields
Crystal analysis is the gold standard for the diagnosis of pseudogout and should be sought whenever possible; chondrocalcinosis on x-ray, by itself, does not diagnose pseudogout.
Although most patients will not have metabolic secondary causes that can be identified, it is worth looking for these, especially checking serum calcium, magnesium, alkaline phosphatase, and iron saturation.
Removal of calcium deposits from joints is not possible, but prophylaxis of recurring attacks of pseudogout, most commonly with colchicine, is often worthwhile.
The coexistence of pseudogout and septic arthritis is not rare, and the infection may stimulate crystal shedding. Culture of the joint fluid must always be done, even when acute pseudogout has been documented.
Consider pseudogout when the femoropatellar joint is more involved than the other two knee compartments on x-ray, or when osteoarthritis is especially severe or occurs in unusual locations, such as the elbow or metacarpophalangeal joints.
When you are considering a diagnosis of gout, but inflammation is present in unusual locations, such as the shoulder or hip, consider pseudogout.
When an elderly individual presents with a single acutely inflamed joint, always consider pseudogout.
Pseudogout is an inflammatory arthritis, with acute and chronic forms, caused by the deposition of calcium pyrophosphate dihydrate (CPPD) crystals within a joint. Chondrocalcinosis, calcified cartilage seen on radiographs, is found in most patients with pseudogout. The presence of CPPD crystals tends to be associated with more aggressive and destructive osteoarthritis.
Systemic conditions predisposing to pseudogout include aging, hyperparathyroidism, hemochromatosis, hypophosphatasia (very rare), and hypomagnesemia. Pseudogout attack may follow parathyroidectomy, thought to be related to the flux in calcium level after the procedure.
Local factors predisposing to pseudogout include trauma, infection of the joint, osteoarthritis and other structural derangements of the joint, and the presence of urate crystals (gout and pseudogout may coexist). Injection of hyaluronic acid preparations for treatment of osteoarthritis has been reported to cause pseudogout attack, presumably by the same “strip-mining” effect wherein crystals within the cartilage are leached off due to a septic process.
Local ion concentrations and inorganic pyrophosphate levels help determine when joint calcification will occur. Calcium and iron levels play a role. When there is matrix supersaturation with inorganic pyrophosphate, it stimulates chondrocalcinosis, and the level rises with age.
CPPD crystals induce an inflammatory response as the crystals re-shed into the joint and undergo phagocytosis by leukocytes. The chronic presence of CPPD crystals in a joint predisposes to joint damage and progressive osteoarthritis.
The genetics of pseudogout include an autosomal dominant pattern that has been described in some populations in which disease begins in early adulthood, progresses rapidly, and is polyarticular. A mutation in the human ANK gene has been shown to lead to a leakage of inorganic pyrophosphate from the chondrocyte to the extracellular matrix, which can eventually lead to articular cartilage calcification.
Osteoarthritis and chondrocalcinosis are both common disorders, and their exact relation has not been fully established. Eight percent of individuals older than 60 years have radiographic evidence of chondrocalcinosis, although most are asymptomatic. The prevalence of chondrocalcinosis increases with age to involve as many as 28% of the population in the ninth decade.
Four different stages are recognized in the clinical evolution of CPPD and pseudogout.
Asymptomatic chondrocalcinosis refers to the radiographic finding of calcified cartilage in patients, without joint complaints. It is very common in the eighth decade and later. Although no attacks of pseudogout occur in this phase, CPPD deposits in joints can lead to cartilage damage and acceleration of osteoarthritis.
Acute pseudogout. In acute pseudogout, there are inflamed joints, often intensely painful. Acute monarthritis occurs in approximately 25% of patients with CPPD. Elderly women are afflicted more often than men. The knee is the most commonly involved joint. Ankle, wrist, and shoulder involvement is also common, and acromioclavicular pseudogout has been described. Attacks are usually self-limiting, lasting several days to several weeks. Surgical procedures, especially parathyroidectomy, and severe medical illness may precipitate acute pseudogout attacks.
Chronic pseudogout can present as pseudo-osteoarthritis or pseudorheumatoid arthritis.
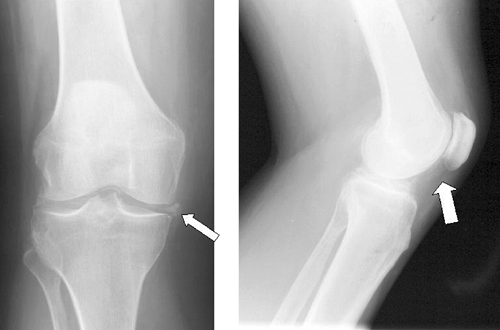
Pseudo-osteoarthritis is the most common pattern of chronic CPPD arthropathy, and is distinguished from primary osteoarthritis by the pattern of involvement of the wrist and metacarpophalangeal joints, and by atypical knee osteoarthritis (Fig. 44-1). CPPD crystals are clearly associated with more severe osteoarthritis and have been found in 60% of knee joints at the time of knee replacement.
Pseudorheumatoid arthritis can present with chronic polyarticular inflammation occurring in up to 5% of patients with CPPD deposition. In some patients, prominent symptoms of fatigue, malaise, and morning stiffness may also develop, making the distinction from rheumatoid arthritis difficult.
Pseudoneuropathic joints can result from the deposition of CPPD crystals in the presence of destructive arthropathy, as seen in Charcot joints. This is usually a chronic relapsing arthropathy with a female predominance of 14:1 and associated with tendon ruptures, especially at the shoulder joints.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree