Total lumbar disk replacement has become a routine procedure in many countries. However, discussions regarding its use are ongoing. Issues focus on patient selection, technical limitations, and avoidance or management of complications or long-term outcomes. A review of the development of this technology, since the development of the first successful implantation of a total lumbar disk prosthesis in 1984, shows an amazing analogy to the history of total hip replacement. This article is a one-to-one comparison of the evolution of total hip and total lumbar disk replacement from “skunk works” to scientific evidence.
Surgical fusion of lumbar motion segments has been widely accepted as a treatment option for degenerative low back pain in patients who fail to respond to conservative therapy (see the article by Fritzell and colleagues for further exploration of this topic). Various fusion methods have resulted in acceptable clinical outcomes for a variety of degenerative pathologies. Improvements in implant technologies (eg, pedicle screws, cages), access techniques (eg, open, mini-open, percutaneous, navigation), as well as the use of osteoconductive (eg, bone substitutes) and/or osteoinductive (eg, bone morphogenetic proteins [BMP]) have facilitated recent advancements in lumbar fusion technology. However, the influence on the expected improvement in surgical outcome remains unclear.
Although it took more than 90 years from the first written report on instrumented spinal fusion to the first randomized controlled study, spinal fusion has always been accepted as a surgical “gold standard.” In 2006, Weinstein and colleagues reported more than 200,000 lumbar fusion procedures were performed annually in the United States between 1992 and 2003. Reoperation rates following lumbar fusion reach 11.9% to 27.4% in follow-up periods between 3 to 15 years. Complication rates up to 70% have been reported, as well as adjacent segment alterations in 31% to 66% of cases. These significant disadvantages, however, have not led to a significant reconsideration of fusion procedures because, for many years, it was the only option surgeons had for the treatment of degenerative low back pain. The worldwide introduction of lumbar total disk replacement (TDR) since the end of the 1990s has produced paradoxic, controversial, and occasionally irrational reactions among surgeons, regulatory institutions, health care insurance companies, and health care providers.
In most European countries, lumbar TDR has become a well established and well accepted therapeutic option (which has not led to an increase in total lumbar surgical procedures) with good-to-excellent midterm results. Several studies have proven the usefulness of this procedure, its noninferiority or superiority to lumbar fusion in selected patients, and its safety when it comes to reoperations or complications. Other studies have shown the cost-effectiveness compared with fusion. The fact that this scientific data are not accepted by health care administrations and insurance companies in some health care systems around the world suggests that these processes are not driven by the needs of the patient. Assuming that it is neither a lack of knowledge nor a lack of common sense, nor ignorance of sound scientific work, the reasons for this are obviously political and economic.
To give an update of prosthetic lumbar disk replacement at the beginning of the second decade of this century, it is helpful to have a look into the past and learn from similar developments. In 2009, there were two anniversaries: (1) 50 years since the first implantation of a prosthetic total hip by Sir John Charnley ( Fig. 1 ) and (2) 25 years since the first implantation of an artificial disk by Buettner and Schellnack ( Fig. 2 ). William H. Harris published an article in 2009 in which he reviewed the first 50 years of total hip replacement (THR). Over the years, five observations were made (“skunk works,” Pasteur’s motto, totally unexpected, research solutions, and the role of alternatives), which more or less characterize the process that THR underwent from the very early attempts and failures to become one of the most successful procedures in orthopedic surgery. In fact, these observations have become a paradigm for what we have experienced in the 25 years since the introduction of lumbar TDR.


Skunk works
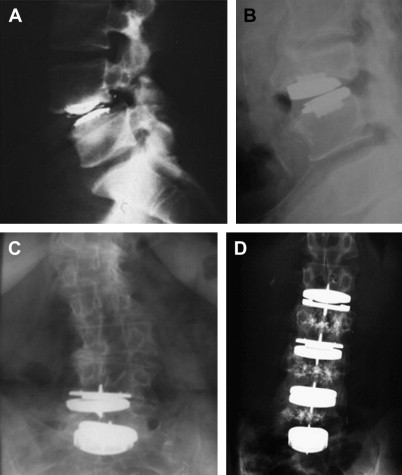
The “very non-traditional effort, which is offline, off-budget, remarkably innovative, driven by creative zealots, uninhibited by failure, and relentless.” This is how Harris characterized “skunk work” in the early days of THR. It was the early failure of Teflon-on-Teflon interfaces, problems with indications, and surgical technique. Looking at some examples of early lumbar disk replacements, we can observe similar “skunk works” such as wrong indications, subsiding of the implant, or spontaneous fusion after disk replacement ( Fig. 3 A, B). Once a technique works, the risk of over-use increases, especially if the indications are not yet standardized or proven. Some typical examples of so-called off-label use of TDR are shown in Fig. 3 C, D. Interestingly, and sometimes fortunately, these applications did not always lead to an unsatisfactory clinical outcome. In summary, there is an analogy between what happened in the early years of THR and TDR. It reflects the surgeons’ attempts to push a technology to its limits.
Pasteur’s motto
“Chance favors the prepared mind” was one of Louis Pasteur’s favorite mottos. It was by chance that Sir John Charnley met Dennis Smith, Chemist at Turner Dental Hospital in Manchester. Smith suggested the use methylmethacrylate for the fixation of hip implants and triggered the development of cemented hip arthroplasty. The history of prosthetic disk replacement is coupled to a historical event—the fall of the Berlin Wall on November 9, 1989. The first total disk implant (SB Charité I) was invented and first implanted at the Charité University Hospital of the Humboldt University in the former German Democratic Republic (East Germany). After the reunification of the two Germanys, Link (Waldemar Link GmbH & Co KG, Barkhausenweg 10,D-22,339 Hamburg, Germany), a well known West German implant manufacturer with long experience in total hip and knee manufacturing, took over the technology and developed the further generations of the SB Charité total disk implant, which then became the first total disk prosthesis on the market.
Pasteur’s motto
“Chance favors the prepared mind” was one of Louis Pasteur’s favorite mottos. It was by chance that Sir John Charnley met Dennis Smith, Chemist at Turner Dental Hospital in Manchester. Smith suggested the use methylmethacrylate for the fixation of hip implants and triggered the development of cemented hip arthroplasty. The history of prosthetic disk replacement is coupled to a historical event—the fall of the Berlin Wall on November 9, 1989. The first total disk implant (SB Charité I) was invented and first implanted at the Charité University Hospital of the Humboldt University in the former German Democratic Republic (East Germany). After the reunification of the two Germanys, Link (Waldemar Link GmbH & Co KG, Barkhausenweg 10,D-22,339 Hamburg, Germany), a well known West German implant manufacturer with long experience in total hip and knee manufacturing, took over the technology and developed the further generations of the SB Charité total disk implant, which then became the first total disk prosthesis on the market.
The totally unexpected
Applying new technologies implies the risk for unexpected adverse events or complications. The pioneers of THR certainly did not expect to create a new iatrogenic disease, periprosthetic osteolysis. Total disks that are not mobile after the implantation are one result that nobody expected. Suboptimal placement of the implant and segmental hyperlordosis due to sagittal imbalance are most probably responsible for this so-called locked-in scenario. It is not clear whether loss of range of motion over time, heterotopic ossifications, or spontaneous fusions, which can occur in a small percentage of patients, are really unexpected. It seems that a segment with a prosthetic disk behaves like a normal segment when it comes to the natural course.
Research solutions—science and randomized controlled trials
Scientific workup in medicine takes time. It took 93 years between the first publication of an instrumented lumbar fusion in May 1908 and the first randomized controlled trial (RCT), which showed scientific evidence for the use of fusion in low back pain. In 2000, 46 years after the description of laminectomy for the treatment of lumbar degenerative spinal stenosis, the first RCT proved its effectiveness. Fortunately, it took just 21 years between the first implantation of a total disk (in former East Berlin in 1984) and the publication of the first Level I, evidence-based data in 2005. The investigators proved “noninferiority” of the Charité disc (DePuy Spine, Raynham, MA, USA) as compared with BAK-cage (Orthofix Inc., Lewisville, TX, USA) supported anterior fusion. Although the success rates were not very convincing, neither were those in the control group after a 2-year follow-up. These results were recently confirmed after a 5-year follow-up period. Two years later, the first RCT was published for the Prodisc Prosthesis (Synthes Inc, Paoli, PA, USA). In a study with 2 years of follow-up, Zigler and colleagues demonstrated evidence for superior results of the Prodisc as compared with 360° fusion.
The Maverick disc (Medtronic, Memphis, TN, USA) was the third implant that received US Food and Drug Administration (FDA) approval. It proved to have less serious device-related adverse events and the patients returned to work earlier compared with stand-alone anterior interbody fusion. This study was the only one that compared two identical approaches (anterior interbody fusion vs TDR). Due to patent violations, this implant is currently not distributed in the United States.
The fourth RCT investigated the FlexiCore Disc (Stryker Spine, Allendale, NJ, USA). In a 2-year follow-up of 67 patients from two sites that were involved in an FDA investigational device exemption (IDE) trial, the results were more or less the same as in other trials proving similar and, by some parameters, superior results compared with fusion. At the time this article was written (December 2010), the IDE study is completed and submitted, but the current status or results are unknown.
Recently, results of a RCT comparing two total disks were presented. The Kineflex Disc (SpinalMotion, Mountain View, CA, USA) was compared with the Charité disc (control group) in 457 patients from 21 sites. Both groups showed significant improvement of clinical parameters, such as the Visual Analog Scale (VAS) and Oswestry Disability Index (ODI) scores, with no significant difference between the groups. This IDE study is also completed and currently reviewed by the FDA.
Although scientific evidence was generated within a relatively short period of time, health care providers and health insurances in some countries still have difficulties accepting TDR as a standard procedure. From a scientific point of view, such attitudes seem to be driven by irrational fears:
- •
TDR could wipe away fusion technology and could lead to a dramatic increase in surgical procedures for low back pain.
- •
TDR would lead to uncontrollable increase of unforeseen sequelae, complications, and adverse events.
- •
TDR would dramatically increase the treatment costs for low back pain patients without improving the clinical results.
In countries where TDR has become a routine procedure, such developments have not been confirmed.
Costs
One concern of health care providers and insurance companies are the costs that are associated with new technology. In 2007, Guyer and colleagues published an economic model of one-level lumbar arthroplasty versus fusion. The investigators analyzed the costs of the implant, hospital stay, operating room time, and so forth, and compared TDR with different types of lumbar fusion procedures (eg, transforaminal lumbar interbody fusion [TLIF], 360° fusion, anterior lumbar interbody fusion [ALIF]) and were able to show that, although the implant costs were similar to the other procedures, TDR is the least expensive type of treatment. In 2008, Patel and colleagues were able to demonstrate that TDR costs are similar to TLIF or ALIF without BMP, that they are lower compared with TLIF or ALIF with BMP, and that they are definitely less when compared with 360° fusion. These results were confirmed as referred to the hospital charges for one-level procedures by Levin and colleagues.
In summary, strong evidence has been published in the scientific literature that supports the safety and efficacy of TDR. Several prospective studies with 2-year to 5-year follow-up, as well as long-term results from outside the United States, prove that TDR results are at least as good as different types of fusion in comparable patient cohorts. TDR is also similar or less expensive compared with various types of lumbar fusion procedures. Although these results have been elaborated with different types of implants (different implant materials, different interfaces, varying kinematic characteristics, etc), there have been no significant differences in the overall clinical outcomes. This suggests that the influence of these different features on the outcome remains unclear.
Influence of Disk Pathology
The first years of TDR were dominated by discussions about the indication for this new type of treatment. The first prospective study analyzed the influence of different indications on the outcome of TDR. Patients suffering from monosegmental or bisegmental degenerative disk disease with low back pain showed significant improvements in VAS and ODI within the first 3-year follow-up. It turned out that there were no significant differences between patients with or without Modic type I changes.
Influence of Previous Surgery
This study did not detect a significant difference in the clinical outcome when comparing patients with disk degeneration with or without previous discectomy surgery. This was confirmed by other independent study groups. However, other investigators reported a negative effect on postoperative outcomes. However, the results from these studies are not comparable with each other owing to differences in selection criteria. Patients with marked epidural scar tissue formation in the spinal canal on preoperative gadolinium diethylenetriaminopentaacetic acid-magnetic resonance imaging, as well as patients with significant facet joint alterations from the previous operation and patients with sciatica, were routinely excluded.
Influence of Age
Similar findings were observed concerning the influence of age on the clinical outcomes of TDR. Several investigators described contradictory results with better outcomes in patients younger or older than 45 years. Other investigators could not prove an influence of age on outcome. There is, however, a general consensus that TDR should not be implanted in patients with reduced bone mineral density. In randomized controlled FDA IDE studies, patients with osteoporosis, osteopenia, or any type of metabolic bone disease were excluded. There is still an ongoing discussion about which patients should have a dual energy x-ray absorptiometry (DXA) scan preoperatively. In a prospective series, DXA scans were performed in all women older than 45 years of age, in all men older than 55 years of age, as well as in all patients in whom either radiographs or the medical history showed signs or risk factors of osteopenia or osteoporosis.
As compared with hip or knee arthroplasty, which can be performed even in elderly patients, TDR can rarely be performed in this age group. Besides the reduced bone mineral density, most of the older patients have other contraindications against TDR, such as spinal stenosis, degenerative deformities (de novo-scoliosis, degenerative spondylolisthesis, etc), as well as symptomatic and advanced facet joint osteoarthritis. All of these factors lead to a more or less natural selection of a younger patient cohort.
Influence of Anatomic Segments and Number of Levels on Outcomes
Several investigators have described similar or even superior clinical results in patients with double level implantations compared with single level TDR. Others have described inferior clinical outcomes in multilevel implantations. In a prospective series, the highest satisfaction rates were observed in patients with TDR at the level L4-5 (90.9% subjective patient satisfaction rate), followed by L5-S1 (78.9%). The lowest satisfaction rates were observed following double-level implantations—L4-5-S1 (65%). The conclusion from these published data is that obviously TDR leads to good results in monosegmental implantations and the results also seem to be acceptable for selected bisegmental cases.
Complications
A wide variation of complication rates has been published in the last 16 years. The overall complication rates range from less than 1% to 39.7%. At least part of these numbers represent the learning curves of individual surgeons. Access and technique-related complications seem to be dominant in most of the papers. The intraoperative and perioperative complication rates in the FDA IDE studies that were performed with the most widely used implants, Prodisc and Charité disc, did not show significant differences between the investigational and the control group. Thus, previously published TDR complications rates are well within the range of complication rates that have been described for ALIF techniques.
Reoperations
To date, there has been considerable concern that TDR might lead to frequent reoperations because of implant extrusions, subsiding, and/or loosening of the implant. These concerns were not confirmed in the studies published until now. The average reoperation rates within the first 2-years following TDR with the Prodisc and Charité disc were 3.7% and 5.4%, respectively. McAfee and colleagues published a reoperation rate of 8.8% following TDR in a series of 589 patients who received a disk replacement with the Charité disc. In another study, the reoperation rate within the first 2 years was 8.1%. These rates do not differ from the ones published after lumbar fusion. Conversely, previously published reoperation rates following lumbar fusion procedures range between 11.9% and 27.4% within the first 3 to15 years postoperative. The vast majority of reoperations in TDR were performed in the early postoperative period and were mainly attributed to faults in either implantation technique or inappropriate indication for TDR.
Sagittal Balance
A postoperative sagittal malalignment is one potential disadvantageous effect of lumbar fusion procedures which may furthermore predispose the segments for adjacent level degeneration. Conversely, previous studies have reported a maintained physiologic sagittal alignment following total lumbar disk replacement procedures. While an improved sagittal alignment is one advantage of TDR over lumbar fusion procedures, a tendency for increased index-level hyperlordosis. In a CT study, Liu and colleagues reported a significant decrease of the facet joint articulation overlap in the sagittal plane and an increase in the facet joint space following an increase in the lumbar disk space. The authors concluded that an inappropriate increase of the disk space height will result in facet joint subluxation. Rohlmann and colleagues similarly reported increasing intersegmental lordosis with increasing implant heights. The authors concluded that great care should be taken in choosing the optimal height and correct position of the implant. Clinical data similarly revealed an increased segmental lordosis and symptoms from the facet joints in a considerable number of patients postoperatively. As a direct result of these studies, the authors have modified their disk replacement strategy over the years by choosing implants with less lordosis (≤6°) and the lowest available implant height (10 mm) for the majority of all patients.
Facet Joint Osteoarthritis
A variety of biomechanical studies have demonstrated increased facet joint pressures, segmental instability at the index level, and altered load patterns with sudden, instead of gradual, load increase in the facet joints following TDR. Radiological studies have demonstrated an increased segmental hyperlordosis with the potential of subluxation of the facet joints. Leivseth and colleagues highlighted the disparity between the prosthesis and the anatomic center of rotation in patients treated with ProDisc II, particularly at the lumbosacral junction. Similarly, Rousseau and colleagues highlighted aberrant centers of rotation following TDR and published possible consequences on facet joint contact forces.
Shim and colleagues reported a 32% to 36.4% rate of degeneration of the facet joints following TDR with Charité III and ProDisc II. Park and colleagues observed progression of facet joint degeneration (FJD) in 29.3% of TDR segments at a follow-up of 32.2 months from grade 1 to grade 2, predominantly in women and 2-level TDR. In accordance with the above-mentioned studies by Shim and colleagues and Park and colleagues, other data similarly reveal a progression rate of FJD in a considerable number of patients (n = 44/220 facet joints; 20%). The severity of FJD was, however, less than expected. A significant difference in FJD rates between the lumbosacral junction and the level L4-5 (23% vs 3.3%) was demonstrated.
In summary, the data suggest that it is more likely a particular cohort of patients with inferior biomechanical compatibility that will experience complaints from the facet and sacroiliac joints and who may stand an increased risk of developing FJD at later postoperative stages. Future studies will have to investigate whether these progressive degenerative changes will predominantly be limited to this particular cohort of patients with unfavorable biomechanical compatibility or if the overall patient population will be affected, which may have consequences for the long-term clinical results of lumbar disk replacement procedures.
Adjacent Segment Degeneration
Huang and colleagues reported a 23.8% incidence (n = 10/42 segments) of adjacent level disease (ALD) following TDR with ProDisc I after a mean follow-up of 8.7 years. However, access to MRI was not available in the study setting. In a retrospective MRI and CT scan study, Park and colleagues published a 4.3% progression rate of ALD in a cohort of 32 patients after a mean follow-up of 32.2 months following TDR with ProDisc II. Conversely, Shim and colleagues reported a 19.4% to 28.6% incidence of ALD in a retrospective MRI investigation of 57 patients after a mean follow-up of 38 to 41 months.
An MRI analysis revealed a low incidence of ALD observed at 10.2% (n = 11/108) of all adjacent levels after an average follow-up of 53.4 months (range 24.1–98.7 months). The degenerative changes were mild and signs of ALD occurred late postoperatively after an average follow-up of 62.5 months. Finally, the occurrence of ALD did not negatively affect the clinical symptomatology. Therefore, these results are in congruence with findings that have previously been published by a variety of independent biomechanical and radiological studies that demonstrated an unloading effect of TDR on adjacent levels compared with fusion procedures.
Prognostic Factors
Predictors of outcome have been investigated for a variety of pathologies and interventions. However, early detection of unsatisfactory late results remains a challenging task. Zindrick and colleagues reported that the existing evidence does not provide any definite conclusions concerning factors that may affect postoperative outcomes. Guyer and colleagues found that the only factor significantly related to the clinical outcome was the length of time off work before surgery. Patients who were off work for shorter durations, or not at all, were more likely to be in the best-outcome group compared with patients who were off work for an extended time before surgery.
Other data demonstrated that baseline ODI and early postoperative outcome parameters (≤6 months) revealed a significant and strong association with the final results following TDR. Although the majority of patients with an early highly satisfactory outcome maintained satisfactory results at later follow-up stages, any significant improvement considered as “highly satisfied” is unlikely in a group of patients who reported an early unsatisfactory outcome. The findings from these studies may be helpful in an attempt to weigh both the patient’s and the spine surgeon’s expectations against any possible realistic achievements.
Sporting Activities Following TDR
Despite its widespread use in predominantly young and active patients, no previous study has addressed possibilities, limitations, and potential risks regarding athletic performance following TDR. Mechanical concerns remain and the implant’s resilience regarding its load-bearing capacity during sporting activities is unknown. A prospective study cohort investigated the ability of TDR candidates to resume athletic abilities postoperatively. The results demonstrate that patients were able to perform a variety of different sporting activities up to the level of competitive sports, extreme sports, and professional athletics. Sporting activity was resumed early postoperatively, within the first 3 (38.5%) to 6 (30.7%) months with peak performance being reached after 5.2 months. Minor implant subsidence was observed in 30% of all patients during the first 3 months with no further implant migration. Therefore, it was not attributed to the sporting activities. No evidence of implant wear was seen in radiological follow-up evaluations. However, owing to the young age of the patients and significant load-increase during athletic activities, concerns about the future of the implant still need to be investigated with larger patient cohorts, longer follow-up evaluations, and modified examination techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







