Chapter 201 Pregnancy Health and Primary Prevention of Adult Disease
 Introduction
Introduction
There is a growing body of evidence suggesting that increased susceptibility to chronic disease in adulthood originates in part during fetal development.1 In utero gene expression is highly sensitive to environmental changes, which can lead to altered function, metabolism, and hormone production.2 In utero environmental exposures alter phenotypic expression via DNA methylation, histone acetylation, messenger RNA expression, and chromatin structure modification. These epigenetic changes occurring in the womb are replicated throughout life, and may even be passed to subsequent generations.
Pregnancy Death Rates and Premature Births
According to the World Health Organization (WHO), the pregnancy death rate has decreased by 30% in the last 20 years. This decline is due to both a worldwide increase in the training of midwives as well as higher quality pregnancy care in higher-risk community health centers and hospitals. Pregnancy deaths are about 36 times greater in a developing country versus a developed country. The main causes of such deaths are severe bleeds after childbirth, infections, hypertension, and improper abortion.3 With more than 540,000 yearly premature births, the United States has a significantly higher rate of infant mortality compared with other developed countries owing to the inadequate pregnancy care received by poor women, increased infertility treatments (which raise the odds of multiple births), and early labor and delivery.4
Weight Gain
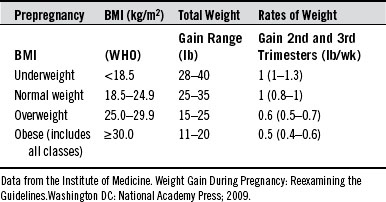
On average, women should expect to gain from 25 to 35 pounds during a normal pregnancy, with a typical 3- to 4-pound gain during the first trimester (Table 201-1). Low weight gain is associated with increased intrauterine growth restriction risk and perinatal mortality. The overall weight gain represents two components: (1) the products of conception (fetus, amniotic fluid, and placenta) and (2) maternal accretion of tissues (expansion of blood and extracellular fluid, enlarged uterus and mammaries, and adipose stores).
Obesity in Pregnancy
Animal studies reveal that high maternal weight or weight gain during pregnancy leads to changes in the hypothalamus, pancreatic islet cells, fat tissue, endocrine physiology, and metabolic pathways affecting homeostatic weight control.5 Excessive gains are associated with gestational hypertension, labor augmentation, high birth weight, cesarean sections, neonatal metabolic abnormalities, and fetopelvic disproportion complicates the risk.6
According to the Centers for Disease Control (CDC), 20% of women are obese at the onset of pregnancy. Babies born to obese women are nearly three times more likely to die within the first month of birth, and stillbirth rates are doubled. Very obese women, or those with a body-mass index of 35 or higher, are three to four times as likely to deliver their first baby by cesarean section versus first-time normal-weight women.7 Weight gains of 6.7 to 11.2 kg (15–25 lb) in overweight and obese women, and less than 6.7 kg (15 lb) will reduce risk. Interestingly, in morbidly obese women, poor weight gain was associated with 30% less use of epidural analgesia and an overall reduction in negative outcomes.8
Thyroid
With normal thyroid function needed for pregnancy success, the need for thyroxine increases in many women.9 Preterm delivery, preeclampsia, hypertension, diabetic complications, placental abruption, miscarriage, and adverse fetal effects have all been reported with hypo- or hyperthyroidism during pregnancy.10
Normal thyroid stimulating hormone (TSH) levels alone do not assure proper thyroid function. Normal TSH accompanying autoimmunity creates a twofold risk for pregnancy loss.11 Although TSH laboratory values range between 0.45 and 4.5 mIU/L, studies suggest that that the upper range cutoff in the pregnancy TSH should be around 2.5 mIU/L.12 Miscarriage and preterm delivery increase with higher levels. Furthermore, women with positive antibodies and no replacement therapy also have higher rates of miscarriage than women with positive antibodies and levothyroxine replacement (13.8% vs. 3.5%).13
One study of 4,123 thyroid antibody−free women found a 3.6% rate of pregnancy loss in women with a TSH level greater than 2.5 mIU/L and double that rate (6.1%) in women with TSH levels between 2.5 and 5.0 mIU/L. Age, obstetric history, and even thyroid function tests did not differ between those who miscarried and those who did not. Statistically, the risk of miscarriage increased by 15% for each elevation of 1 mIU/L in the TSH level. Preterm and very preterm delivery risks did not differ with TSH levels.14
Preeclamptic women are more likely to have hypothyroidism before conception or in late pregnancy. After the onset of preeclampsia, predelivery TSH increased 2.42 times above baseline versus 1.48 times in controls. Toward the end of pregnancy, women with preeclampsia had, in general, much higher levels of TSH than women with no history of preeclampsia.15
Flu Shots and Vaccines
Vaccination is recommended in several countries, and no studies demonstrate an increased risk of maternal or fetal adverse effects with inactivated, unadjuvanted shots.16,17 The U.S. Advisory Committee on Immunization Practices recommends the influenza vaccine in pregnancy, citing increased risks of flu complications in pregnant women. There is a 41% decreased incidence of flu in infants born to vaccinated women as well as a 39% decrease in influenza-like illness and hospitalization. Infant antibody titers were significantly higher with vaccinated pregnant mothers. It should be noted that one of the study authors received grants from three companies that manufacture influenza vaccine.18
Newer vaccines, such as the influenza/H1N1 vaccine, now include adjuvant, which may induce a stronger immune response in pregnant individuals, because they have been shown to be more sensitive to proinflammatory stimuli than those who are not pregnant.20 An adjuvant (Box 201-1) is a substance that, when administered in combination with a specific antigen, enhances the immune response against the antigen when compared with use of the antigen alone. Many pregnant women were vaccinated with the adjuvanted H1N1 pandemic influenza vaccine at a time when its safety had not been tested.20
The CDC Advisory Committee on Immunization Practices in pregnancy21 supports giving pregnant women a number of vaccinations during pregnancy and lists only two as contraindicated. These are listed in Box 201-2.
 Lifestyle Factors
Lifestyle Factors
Hydration
Vital for a healthful pregnancy, appropriate fluid intake hydrates all body cells and provides a normal 40% volume expansion in the pregnant woman.22 About 32 ounces of fluid surround the baby as amniotic fluid, which is replaced every third hour. Insufficient fluid intake can cause constipation, preterm labor, temperature dysregulation, cystitis, miscarriage, and fatigue. Proper hydration is also important for subsequent breast milk production and flow.
Energy Needs and Diet
Pregnancy is a demanding anabolic state that hormonally redirects nutrients to maternal reproductive tissues and the developing infant.23 Pregnancy leads to a modest increase of energy needs on the order of 375, 1200, and 1950 kJ daily for the first, second, and third trimesters of pregnancy respectively,24,25 which can normally be met by modest increases in consumption of a balanced diet. The 2002 Dietary Reference Intake Report set a minimum level of 175 g carbohydrate per day for pregnancy, which includes an additional 33 g carbohydrate for fetal brain development and functioning over nonpregnant women. There is no indication that recommendations for dietary total fat intake, expressed as a percentage of energy intake, need to differ from those for nonpregnant women.26 With micronutrient deficiencies common during pregnancy (even in well-fed populations),27 it is important to consider a prenatal multivitamin along with a healthful diet.
Under- and Overnutrition
Undernutrition during pregnancy and low birth weight epigenetically increase the risks of diabetes and cardiovascular disease in adulthood.28 In birth cohorts conceived during the height of the Dutch Hunger Winter famine of 1944–194529 and the severe China famine, from 1958–1961,29,30 fetal effects included higher weight and waist circumferences, higher adult BMIs, increased risk of hypertension, impaired glucose tolerance,31 increased risk for cardiovascular disease32 and diabetes,33 and a twofold increase in the incidence of schizophrenia34 and both mood and schizoid personality disorders.35
Excess and unbalanced food choices will also have a negative impact on the health of the offspring via high birth weight, which increases the risks for obesity and diseases such as cancer and asthma later in life. It is appropriate for a baby to be born with some adipose tissue, but excess fat is indicative of abnormal fetal development.5
Healthful dietary changes after a baby is born may not be able to reverse the original fetal insult. Pregnant rats fed an unhealthy diet are more likely to have offspring that develop breast cancer, likely due to the in utero effect on mammary remodeling. Even when daughters eat healthily, their disease risk is still not ameliorated. Interestingly, daughter rats with grandmothers who ate a fatty diet were even more at risk. In one study at 20 weeks, 50% of the rats whose grandmothers ate a normal diet developed breast tumors, whereas 80% of rats with two grandmothers fed a high-fat diet grew tumors and 68% of the rats with just one such grandmother developed cancer. These animal studies strongly suggest that a fatty diet may cause epigenetic DNA modifications that can be passed on to future generations.36
The brain and nervous system are also subject to the epigenetic changes of a high-fat diet. One study looked at three different pregnancy diets: the first diet was high in saturated fat, the second was high in unsaturated or “trans” fat, and the third was a control low-fat diet. At weaning and in adulthood, pro-inflammatory cytokine expression was strikingly increased in the periphery and hippocampus following a bacterial challenge in the high-fat and trans-fat groups compared with controls. Anxiety was significantly increased in fat-exposed offspring, particularly males, and there was a negative effect on learning. These effects were all observed in adulthood, even after the pups were placed on standard chow at weaning, suggesting that these outcomes were programmed in utero. The findings suggest that maternal diet will program brain changes that begin before birth and that these cannot be reversed in the offspring with a change in diet.37
Healthy Foods
Fruits and Vegetables
Pregnant women require more fruits and vegetables than usual because of the extra demands on the body. One study of 1034 women reported dose-dependent reductions in urinary tract infection with an increased intake of both fruits and vegetables, not either alone.38 The recommendation is seven servings per day of fruits and vegetables during pregnancy. A serving size for fruit or vegetables is one half cup. Less dense greens like spinach and lettuce have a serving size equal to one full cup.39
Folic Acid−Containing Foods
Given the well-known association between deficiency of folic acid during the prenatal period with spina bifida and other neural tube defects,40 pregnant women should be encouraged to eat foods specifically higher in folic acid. Foods containing excellent to good sources of folic acid are fortified grains, spinach, lentils, chick peas, asparagus, broccoli, peas, Brussels sprouts, corn, and oranges. However, research suggests that diet alone is unlikely to provide levels similar to those from folate-multivitamin supplementation.41
Apples
In two birth cohort studies of children, maternal intake of apples or apple juice had a protective effect on the children, with a 40% decreased rate of wheeze and about a 50% lowered risk of asthma at age 5.42,43 Apples have a powerful phytochemical content, which includes flavonoids, isoflavonoids, and phenolic acids. Because apple juice is a high-sugar food, whole apples are advised.
Vegetarian Diets
Although vegetarian diets are associated with healthier pregnancy weight,44 strict vegan diets have been reported to cause low pregnancy vitamin B12 status, anemia, and delayed infant development.45 The American Dietetic Association (ADA) proffers that properly planned vegetarian or vegan diets can be “healthful, nutritionally adequate, and may provide health benefits in the prevention and treatment of certain diseases.” The ADA also suggests that well-planned vegetarian diets are appropriate for individuals during all stages of the life cycle, including pregnancy and lactation.46
Soy
Soy is a staple in many vegetarian diets. The use of soy during pregnancy is controversial. A Danish analysis suggests that a pregnancy diet lacking fish and meat was associated with a more than fourfold increased risk of hypospadias,47,48 possibly due to increased soy intake. Suspected estrogenic effects49 may disrupt male masculinization through interference at the pituitary-gonadal axis.50 Study of animal perinatal genistein exposure resulted in alterations in the male reproductive system (i.e., smaller anogenital distance and testis size, delayed preputial separation, lowered testosterone). Maternal exposure to the daidzein in rats during pregnancy had a masculinization effect on female offspring in regard to memory and social behavior.
Generations of Asian cultures have consumed soy. At this point, there is no clear evidence of reproductive damage or untoward effects in humans.51 As a prudent measure, it may be helpful to balance soy protein with other sources of protein.
Fish
Eating fish during pregnancy can support future brain development and reduce the risk of asthma and atopy in offspring by 40%; it can also help to prevent postpartum mood disorders in the mother.52 As a rich source of the essential long-chain n-3 (omega-3) polyunsaturated fatty acids (PUFA), fish provides structural and physiologic support for neurologic, immune, and cardiovascular system development. In pregnancy, the desired minimum intake of 200 mg docosahexaenoic acid (DHA) per day26 can be reached with the consumption of one to two portions of sea fish per week, including oily fish such as herring and salmon.26,53
Unfortunately, fish contributes significantly to dietary exposure to methylmercury, dioxins and polychlorinated biphenyls, brominated flame retardants, camphechlor, and organotin.54 Bioaccumulative contaminants tend to concentrate in larger fish.
Mercury
The greatest susceptibility to the critical contaminants methylmercury and the dioxin-like compounds occurs during early development. Methylmercury is particularly toxic to the developing brain and may also adversely affect child growth.55–57 A woman can effectively decrease methylmercury levels in her body by reducing the intake of contaminated foods in the months prior to and during pregnancy. Compared with other foods, salmon is relatively low in mercury as well as in levels of 18 other trace metals; however, the levels are three times higher in wild salmon than in farmed. Total mercury intake from both is lower than from many other foods58 (Box 201-3).
Polychlorinated Biphenyls (PCBs)
Although PCB use ceased in the 1970s, virtually all samples of human blood, fat, or breast milk show some detectable PCBs.59 They are found at the highest levels in fish and inorganic butter; moreover, farmed salmon contains significantly higher levels of PCBs than wild varieties of the fish. It was therefore concluded that eating farmed Atlantic salmon, “may pose health risks that detract from the beneficial effects of fish consumption.”60
The ingestion of PCB-contaminated fish before and during pregnancy can contribute to low birth weight, small head circumference, earlier birth, a depressive response in the infant, and impaired vision and memory. At age 11, children who had been exposed to PCBs had thrice the risk of low verbal IQ and twice the lag in reading comprehension/attention issues as children who had not been so exposed.61
The U.S. Food and Drug Administration (FDA) and Environmental Protection Agency recommend that pregnant women and youngsters eat no more than two servings of salmon or other low-mercury fish each week.62 However, the benefits of eating fish may outweigh the risks. It may be prudent to have pregnant women focus on fish with lower mercury and PCB content and to avoid eating the skin and fat typically found along the ventral, lateral, and abdominal areas, where PCBs are most heavily deposited.
Flaxseed
Rat studies using a high percentage of dietary flax in pregnancy have shown lower birth weights, female offspring with shortened anogenital distance, greater uterine and ovarian relative weights, lighter body weight at puberty, as well as lengthened estrous cycle and persistent estrus; the males had reduced postnatal weight gain, greater sex gland and prostate relative weights, and delayed puberty.63 An analysis of 3191 study subjects found that exposure to flax oil during the last two trimesters of pregnancy quadrupled the risk of preterm birth. The authors of this study were not clear on doses or whether the effect of flax on prematurity is explained by a phytoestrogenic effect.64
Caffeine
Caffeine intake, most often in the forms of coffee or black and green tea, is commonplace during pregnancy. Although rat studies using 24-cup human-equivalent doses of black tea found no changes in pregnancy outcomes,65 a study of 1063 pregnant women who consumed 200 mg/day or more of caffeine (the amount in 10 ounces of coffee or 25 ounces of tea) revealed a doubling in the risk of early miscarriage (12.5% to 24.5%). The increased risk was associated with caffeine itself and was separated from confounders such as smoking or advanced maternal age.66 An 8-year analysis of 3346 case infants and 6642 controls found no teratogenic consequences of maternal caffeine intake.67,68
Cocoa/Theobromines69
Having increased in per capita intake over the last four decades, cocoa and chocolate have enjoyed popularity and perceived health benefit with little pregnancy outcome information. One large study has nonconclusively suggested that maternal prenatal intake of cocoa could be associated with both hypospadias and testicular cancer in male offspring. Moreover, a study comprising a 2291-woman cohort found that theobromine concentrations in cord serum from five or more servings of chocolate was inversely associated with preeclampsia when compared with women who consumed less than one serving of chocolate weekly.70 Another study found no correlation.71
Stevia
Stevia is a small perennial shrub that yields a white crystalline compound (stevioside) commonly used as a natural sweetener. With no calories, it is over 100 to 300 times as sweet as table sugar. Animal studies suggest no effect on growth or reproduction.72–74 Although no human trials are available, the FDA rates stevia as generally recognized as safe (GRAS).
Peanuts
About 1% of the population reports peanut or tree nut allergy.75 Studies have found that the babies of mothers who ate a greater number of peanut products during pregnancy were more likely to test positive for peanut allergies (showing up as allergic factors in the blood).76 What is particularly interesting in this study is that all sensitized infants also tended to have either milk or egg allergy and/or significant eczema. It is possible that this underlying inflammatory response may cause increased reactivity to peanut. Although not conclusively known, maternal peanut consumption during pregnancy may accelerate child peanut sensitization.
Sleep
Anecdotally thought of as a ‘preparation for life with the newborn,” interrupted sleep during pregnancy is a challenge to the health of the mother-to-be as well as to her expected child. Pregnancy itself will also affect the quality of sleep, with difficulty in assuming the usual sleep positions, discomfort from fetal movements, cramps, and poor sleep quality, with the latter being influenced by the previous complaints. Pregnancy is associated with some specific sleep disorders, such as obstructive sleep apnea and restless legs syndrome. Decreases rapid-eye-movement sleep during the last trimester can be explained by progesterone and estrogen alterations.77
Animal pregnancy studies show that sleep deprivation affects child renal development negatively.78 Human sleep deprivation (less than 8 hours per day) during pregnancy risks a 3.8 times increase in first-trimester miscarriage,79 impairs the mother–infant relationship, and promotes postpartum depression.80
Exercise
Recommended by all major health departments as well as the American College of Obstetricians and Gynecologists (ACOG), the Department of Health, and the U.S. Department of Health and Human Services, exercise during pregnancy has shown improvement in positive well-being, moderate effects on psychological distress, a large reduction of fatigue in the second trimester,81 as well as lower miscarriage79 and depression risk82 (Table 201-2).
TABLE 201-2 Recommendations for Exercise During Pregnancy
Recommendations based on the following:
Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry 2009;31(5):403-413.
American College of Obstetricians and Gynecologists (ACOG). Exercise during Pregnancy. American College of Obstetricians and Gynecologists, Washington, DC; 2003.
Department of Health (DH-UK): The Pregnancy Book 2007. London: Department of Health; 2007.
U.S. Department of Health and Human Services (HHS-US). 2008 Physical Activity Guidelines for Americans. Washington, DC: U.S. Department of Health and Human Services; 2008.
Commonsense physical activity recommendations include avoiding contact sports, engaging in low-impact activities, wearing an athletic support bra, drinking ample water, and eating a snack 30 minutes before exercise to assure adequate blood sugar levels.
Studies suggest that regular exercise during pregnancy can have a positive effect on a baby’s birth weight without putting the pregnancy or child at risk. Regular exercise was associated with lower birth weights (within the normal range) and reduced cord concentrations of growth-related peptides, suggesting an influence of exercise on endocrine regulation of fetal growth.83
One interesting case of exercise during pregnancy is that of Paula Radcliffe, an English champion long-distance runner. Ms. Radcliffe ran throughout her pregnancy, avoiding heart rates above 160 beats per minute, and she agreed to uterine imaging every month after month five. For the first 5 months, she ran twice a day, 75 minutes in the morning and 30 to 45 minutes in the evening. After month 5, she ran 1 hour in the morning and then used a stationary bike at night. She ran up to the day before she gave birth to a healthy baby. Twelve days after her daughter’s birth, she started running again.84
Alcohol
Fetal alcohol syndrome is the leading cause of mental retardation in the United States. Sources suggest that earlier gestational exposure to alcohol, often during the time before a woman has become aware of her pregnancy, may be most detrimental. According to studies, 60.2% of women said they did not drink during their pregnancies, and 25.9%, 5.5%, and 2.5% were categorized as light, moderate, and heavy/binge drinkers, respectively.85 Social factors such as being single or divorced, and intimate partner violence increased alcohol consumption risk.86
Smoking
Smoking during pregnancy is among the leading preventable causes of adverse maternal and fetal outcomes.87 About 37% of women smoke before becoming pregnant, and 27% continue to do so during their pregnancies. Pregnancy smoking leads to fetal growth restriction, retroplacental hematoma, increased preterm birth, shorter fetal crown-to-rump lengths, placenta previa, and death in utero.88,89 Smoking cessation is an important goal for the woman thinking about pregnancy, and it is urgent in pregnant women.90
Cannabis
Cannabis is the most frequently used illicit drug among pregnant women, and prenatal cannabis exposure is associated with fetal growth restriction, decreased birth weight, reduced length, smaller head circumference, and newborn cognitive, motor, and emotional issues.91–93 Increases in offsprings’ later tobacco use and adolescent psychosis have also been noted.94
Betel Nut
The fourth most commonly used drug in the world after tobacco, alcohol, and caffeine is the betel nut. It is the seed of the Areca palm, which grows in much of the tropical Pacific, Asia, and parts of east Africa. It is customarily wrapped in betel leaves and chewed. In Taiwan, 23.7% of aboriginal women were found to have chewed betel during pregnancy versus almost no nonaboriginals.95 Studies in mice have shown that betel alkaloids cause skeletal immaturity,96 and cases of human newborns showing withdrawal symptoms are documented.97
Stress and Mind-Body Modalities
High stress triples the risk of pregnancy hypertension, causes adverse birth outcomes, and doubles preterm delivery risk.98 Whereas low and moderate anxiety in the first or early second trimester did not significantly affect birth outcomes,99 anxiety in the second and third trimesters is linked to smaller infant size, lower Apgar scores, increased obstetric complications, lower birth weight, and shorter birth length, after adjustment for confounders, as well as shortened gestational age.100,101
Stress during pregnancy also alters regulation of the fetal hypothalamic-pituitary-adrenal (HPA) axis,102–105 with altered hippocampal glucocorticoid receptor density and sensitivity. Maternal anxiety or depression during pregnancy is linked with HPA axis overreactivity and higher cortisol in infants and children.106–108 Impaired functioning of the HPA axis is associated with metabolic syndrome,109 fibromyalgia, depression,110 and posttraumatic stress disorder111,112 and likely increases the susceptibility of offspring to stress-related disorders.
Mind-body modalities may hold the greatest promise in balancing HPA axis function. There is evidence that pregnant women benefit from mind-body therapies used in conjunction with conventional prenatal care. In a meta-analysis, progressive muscle relaxation was the most common intervention used in pregnant women. Other studies used a multimodal psychoeducation approach or a yoga/meditation intervention and found modest evidence for the efficacy of mind-body modalities during pregnancy. Treatment group outcomes included healthier birth weight, shorter labor, fewer instrument-assisted births, and reduced perceived stress and anxiety.113
Sunlight
Several studies have shown that the month of birth can affect the birth weight and height of children. The mechanism of this phenomenon is not fully explained, but the importance of hours of sunlight may play a role. An evaluation of 10,631 neonates found that higher temperature, more hours of sunlight, greater rainfall, and lower humidity are associated with greater birth length.114 Prenatal sunlight is one of the most significant determinants of height.115 These findings signify that better nutrition and more active lifestyles of pregnant women, with a longer warm period, may have beneficial outcomes.
Conversely, diseases in offspring may be encouraged by minimal sun exposure. An Australian study reports that low maternal exposure to sunlight during the first trimester of pregnancy may increase the risk of offspring later developing multiple sclerosis.116 Other autoimmune diseases may also play a role this relationship.
A likely factor in sunlight benefit is the role of vitamin D for both healthy pregnancies and healthy offspring. Pregnancy is a vulnerable time for vitamin D deficiency because of increased physiologic needs and reduced maternal outdoor activity.117 Although there are some vitamin D−rich foods, their benefit is minimal in comparison with sun exposure. The importance of vitamin D in pregnancy is discussed further later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree