| 6 | Plastic and Reconstructive Surgery |
General Comments
Primary Treatment and Prevention of Post-traumatic Osteomyelitis
Insufficient primary debridement of fractures with soft-tissue damage, delayed revascularization, and extensive exposure of the bone for fracture stabilization result in a vicious cycle, which is promoted and sustained by ischemia of the wound edges, postprimary infections, and compartment syndromes. If the clinical signs of a beginning infection are recognized at a later stage or not at all, this leads to a loss of soft tissue caused by infection, which spreads to the exposed bones. This results in an increase in surgical interventions, treatment days, inability to work, and psychological problems.
Therefore, systematic treatment strategies are required to help identify and remove potential sources of error.
The extent of soft-tissue trauma is often underestimated in open injuries due to the visible destruction of soft tissues in defined areas. The actual soft-tissue damage is far more extensive.
There is only a gradual difference between closed and open soft-tissue damage. Initially in closed soft-tissue trauma, only indirect signs indicate the severe tissue damage. Dislocations, diastasis of fragments, severe lateral displacements, and comminuted fractures, as well as foreign bodies and air inclusions identified in radiographs indicate massive disintegration of the tissue structures. Underestimation of the damage, failure to treat sufficiently, traumatic surgical techniques, and increasing disturbance in perfusion caused by forced wound closure are classic triggers that magnify the primary injuries. These complex injuries require an all-encompassing concept in which soft-tissue reconstruction takes precedence.
NOTE
In fractures with soft-tissue damage, treatment of the soft-tissue problem takes precedence. Fractures generally heal without complications when the soft-tissue defects are not infected and have good perfusion.
NOTE
Effective management of complications is only possible with optimal interdisciplinary cooperation.
NOTE
The actual extent of soft-tissue damage can only be identified during careful intraoperative exploration.
NOTE
If, following planned serial debridements, final wound closure succeeds within the first 8 days after the trauma, this significantly reduces the rate of posttraumatic osteomyelitides.
Cleaning out the Infection and Covering the Soft-Tissue Defect
Basic surgical training teaches that only wounds with smooth edges and without contusion zones, recesses, and foreign-body contamination can be expected to heal primarily after adaption without tension. Friedrich’s (1898) and von Reyher’s (Lehnhardt et al. 2002) recommendations based on empirical and clinical observations have since been scientifically confirmed. The essential parameters of wound healing, like angiogenesis in soft tissues and bones, cell proliferation, collagen synthesis, protein production, reepithelization, and cellular hormone activity, depend on a constant supply of oxygen and nutrients. Moreover, sufficient elimination of bacteria by means of increased macrophage activity can only be expected with physiologic oxygen concentrations in the tissues.
The surgeon supports these natural functions by performing primary radical excision of devitalized or insufficiently perfused bones and soft tissues.
If the extent of the resulting defect does not permit primary wound closure (Figs. 6.1 and 6.2), local and microvascularly attached flaps are used to provide complete biologic coverage with restitution of the natural skin barriers.
If large open wounds with exposed bones remain, granulation tissue usually loses the battle against bacterial contamination and infection. In particular, bradytrophic tissues, edematous soft tissues, and bone fragments separated from their origins cannot recover their normal perfusion. The well-known surgical concept of restraint (wait until demarcation appears) in chronic peripheral circulatory disturbances (peripheral arteriovenous disease, diabetes mellitus) is not acceptable under these circumstances.
NOTE
Neither antibiotics nor local enzymatic treatment can replace radical surgical debridement.
NOTE
If it is not possible to interrupt the beginning vicious cycle consisting of primary tissue damage, local hypoxia, infection, and secondary tissue damage early by excising tissue and providing final skin closure, this can result in far more extensive damage and even loss of the affected part of the extremity.
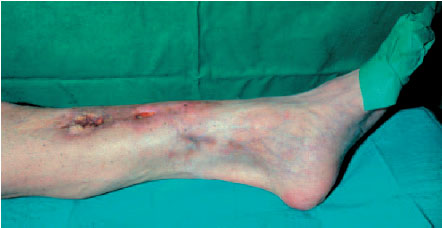
Fig. 6.1 Typical finding in fistulous osteomyelitis of the tibia with chronic secretion.
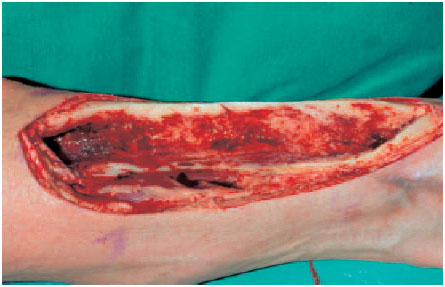
Fig. 6.2 Operation site after radical debridement of the tibia and the soft tissues.
NOTE
Despite a multitude of available microsurgical reconstructive procedures, amputation remains a therapeutic option.
Depending on the damage, one must often exchange preservation of the limb for loss of function. If amputation is unavoidable, transfer of distal portions of the amputated extremity onto the stump for improvement of functional rehabilitation with a prosthesis should be discussed.
Problems accompanying microvascular tissue transplantation:
• Exaggerated indication
• Vascular anastamosis in the infected region, position of the vessels (kinking)
• Wound closure under tension
• Strangulating dressings (swelling, compression due to incorrect positioning)
• Delayed revision
• Unnecessary lengthening of the operation by individual surgeons (the standard is two teams)
Owing to tactical and technical improvements, the success rate of microvascular transplants is approximately 95%. Analysis of failures has identified the causative problems listed here. Aside from the choice of appropriate procedure for the patient’s specific local problem, tactical errors and delayed revisions constitute the main causes. Timely secondary interventions can usually preserve transplanted flaps.
Since microvascular techniques make up only one, albeit a very essential, factor in the treatment of chronic osteomyelitis, interdisciplinary cooperation is necessary to reduce the error rate in general management.
The most effective prophylactic and therapeutic measures for chronic osteomyelitis depend on early plastic surgery of the soft tissues following radical debridement for success. Moreover, these are ideal prerequisites for secondary osteoplastic interventions and measures to improve function.
Problems in the treatment of soft-tissue damage on the extremities:
• Evaluation of the wound: underestimation of the extent of damage
• Evaluation of the peripheral perfusion: delayed revascularization, failure to slit the compartment in time, unfavorable incisions
• Instead of programmed serial debridement: waiting for demarcation, leaving wound recesses and bone cavities
• Insufficient interdisciplinary cooperation
NOTE
Gaps in bone or soft tissues are preferable to continued infection. Petechial bleeding indicates adequate perfusion in the medullary cavity and sufficient debridement. In chronic wounds existing longer than 7 years, biopsies of the edges should always be taken to exclude a carcinoma of the scar tissue.
Radical, extended wound debridement is a fundamental prerequisite for long-term eradication of infection. In a clinically manifest infection, any devitalized bone fragment is to be considered contaminated with bacteria and removed. Fistulous tracts can be filled with blue dye. Compromises cause recurrences.
Unstable fracture fixation disturbs bone healing, and stable fixation accelerates bone consolidation. The treatment principle is to remove nonstabilizing material and to leave osteosynthetic material which is stably anchored. External fixation normally fulfills all requirements for renewed fracture fixation.
NOTE
Bone and soft-tissue defects must be filled in with well-perfused, vital tissue and securely closed.
Flaps
In the presence of an acute infection, the first step is to clean out the focus of infection by means of debridement. After the acute signs of infection and concomitant edema have abated, a second debridement increases the certainty of a successful outcome. The defect should only be covered after several sequential cleansing operations have been performed (lavage in stages).
Several different principles are available for surgical coverage of the defect (Fig. 6.3).
Direct wound closure is only seldom possible—in small defects and areas with stretchable surrounding soft tissues—and should be completely tension-free.
Skin Grafting
Since revascularization is of central importance in surgical toilet, skin grafting alone has declined in importance. Only fascia or muscle flaps ensure a distinct improvement in perfusion. Skin grafting alone should only be performed in exceptional cases in aged patients with impaired vascular systems after granulation tissue has sufficiently covered the bare site. The introduction of modern continuous drainage methods (e.g., Vacuseal, K.C.I., Wiesbaden, Germany) improves the outcome of procedures combining culturing granulation tissue and secondary split-skin grafting for parts of extremities which are not subjected to mechanical stress. A well-vascularized and noninfected recipient site is the prerequisite for a successful split-skin graft. In special cases coverage can also be achieved with the aid of Integra artificial skin (Integra Lifescience, Mainsborough, NJ, US).
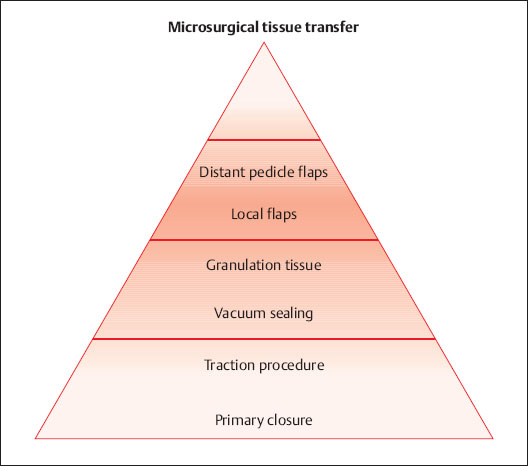
Fig. 6.3 Reconstruction ladder for defect coverage.
NOTE
Reverdin’s (pinch) grafts with small, mixed islands of skin have been completely abandoned due to their vulnerability and unaesthetic appearance, especially at the donor sites.
NOTE
The average thickness of a split-skin graft should be approximately 0.3 mm.
Free skin grafts, especially as mesh grafts, are considered simple and sure procedures, and the rate of healing is high even if the receiver site is slightly infected. Aside from lacking a revascularization effect and unsatisfactory cosmetic results, decreased ability to withstand stress, especially on mechanically vulnerable areas, are large disadvantages. Following slight traumas, these areas tend to develop pressure sores and cracks (Fig. 6.4).
Free, full-thickness skin grafts require good perfusion and lack of infection in the recipient region and are used in particular for the correction of large scars. The groin offers a good harvest site, where donor sites of up to 28 × 8 cm corresponding to an abdominal flap can be closed with a primary suture.
Split-skin grafts can be harvested in various thicknesses.
Sufficient germinal material remains, especially in the hair follicles, for spontaneous healing of the donor site. Harvesting is ideally done on the buttock or thigh. A mesh (accordion) graft is usually used to increase the size and ensure drainage of secretions. The donor site can be dressed with simple fatty gauze; however, use of an occlusive foil is preferable, which leads to faster healing in a liquid milieu and causes less pain.
A split-skin graft receives nutrients by means of diffusion and perfusion (plasmatic imbibition) within the first 3 days if the recipient area has sufficient blood supply. This is followed by capillocapillary docking (inosculation) and capillary proliferation, developing rapidly in muscle and fascia and slowly in the periosteum and tendon sheath. There is no angiogenesis in bones without periostea and exposed tendons.
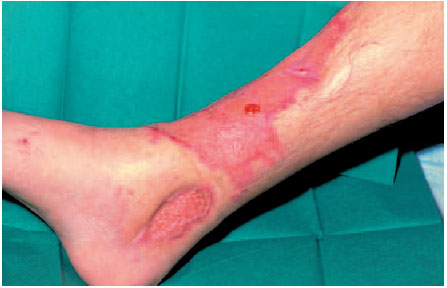
Fig. 6.4 Insufficient soft-tissue coverage in a split-thickness graft transplanted directly over the bone.
The graft is secured with single sutures or staples. To prevent shearing forces, apply a foam tie-over pressure bandage.
The first dressing change takes place after approximately 5 days because the graft is then adherent. If the recipient site is infected, the dressing should be changed earlier if odor indicates a complication.
NOTE
Performing careful hemostasis prior to placement of the skin graft prevents the development of a hematoma, which could cause the graft to fail due to lack of direct contact with the recipient site.
Skin Flaps
If the recipient site is not suitable for a split-skin graft or if this kind of coverage would produce a poor functional result, a flap is indicated.
McGregor and Morgan’s classification is used for practical reasons (Krupp 2002). The determining factor is the form of perfusion in the flap. Accordingly, skin-fat flaps with vascular circulation from the subdermal and dermal plexes (random pattern flaps) are differentiated from flaps with axial perfusion (axial pattern flaps).
NOTE
The decisive factor is the perfusion of the flap. Accordingly, skin-fat flaps with blood supply from the subdermal and dermal plexes (random pattern flaps) are differentiated from flaps with axial perfusion (axial pattern flaps).
The first group is raised without particular attention to the vascular anatomy. They usually contain 1-2 or more angiosomes. An angiosome is an area of skin perfused by an anatomically defined blood vessel. For this reason the relationship between the length and the width of the pedicle should not exceed 2:1, otherwise the perfusion of the periphery of the flap may be disturbed.
In axially perfused skin flaps the incision is based on the course of the anatomically identified vascular system. The relationship between the length and the width of the pedicle can be up to 5:1, depending on the cross-section of the supplying vessel. A gain in length can also be achieved by a properly prepared incision around the vessel. Fasciocutaneous or perforator flaps represent the further development of the principle of axially defined blood supply of soft-tissue flaps. The principle is the dissemination of perforating vessels above and immediately below the deep facia and the muscle septa (e.g., radial flaps).
The form of flap perfusion plays a key role. A major pedicle has blood vessels that can nourish the flap alone. Additional smaller vessels, which cannot nourish the flap alone, are referred to as minor pedicles. There are, of course, flaps with several major pedicles, that is, each of these pedicles could nourish the flap alone. Some minor pedicles that are able to nourish the flap together if the major pedicle fails are called secondary, segmental pedicles. The localization and distribution of major and minor pedicles of individual muscles in the human body is very consistent.
NOTE
Random pattern flap: length to width = < 2:1. Axial pattern flap: length to width = <5:1.
NOTE
Always attempt to leave the flap on its major pedicle.
Muscle Flaps
Muscle flaps provide better coverage of exposed bones. Therefore, a muscle or a combined skin-muscle flap is preferred, especially in chronic bone and soft-tissue infections. The choice of flap is very important. The expected defect at the harvest site and the surgical stress for the patient must be weighed against covering the defect and also the risk of the graft not taking. This must be discussed in detail with the patient. Fundamentals for the choice of flap are its size, its constituent parts (muscle, musculocutaneous, fasciocutaneous, osteomusculocutaneous, etc.), and its blood supply. The patient’s biologic condition, concomitant illnesses, like peripheral arteriovenous disease, post-thrombotic syndrome, and disturbances in lymph drainage, must be considered. If a local flap is chosen, the expected rotational arch is also a criterion.
NOTE
Precise knowledge of the blood supply of the individual muscles is a fundamental prerequisite for the successful performance of this operation.
Classification of flaps according to their arterial blood supply (Mathes and Nahai):
• Type I: One vascular pedicle (dorsalis pedis flap, gastrocnemius muscle, tensor of facia lata muscle, vastus lateralis muscle)
• Type II: One dominant (major) and several small (minor) vascular pedicles (e.g., abductor digiti minimi [foot], gracilis, soleus, and rectus femoris muscles)
• Type III: Two dominant (major) vascular pedicles (gluteus maximus, rectus abdominis, and semimembranosus muscles, etc)
• Type IV: Segmental vascular pedicles (sartorius, tibialis anterior, and flexor hallucis longus muscles, etc)
• Type V: One dominant (major) vascular pedicle and secondary segmental vascular pedicles (latissimus dorsi, pectoralis major muscles, etc)
NOTE
An Allen test must be performed before a radial flap is removed from the lower arm to ensure sufficient perfusion of the hand from the ulnar artery. If a disturbance in perfusion of the thumb occurs after removing a radial flap, a vascular replacement with a venous interposition graft is indicated (great or small saphenous vein).
Microsurgically Attached Flaps
Preparatory examinations are required for free flaps. In addition to clinical examinations including the vascular status at all levels and Doppler ultrasound of the vessels, if there is doubt about the blood supply at the recipient site, angiography must be performed for clarification and to plan possible anastamoses or required alternatives.
A vascular operation or a temporary vascular loop must rarely be performed before the planned intervention. If conditions are insufficient for an anastamosis (e.g., severe peripheral arteriovenous disease), a bypass can be performed. The improved perfusion of the lower leg creates the necessary prerequisites for a microsurgical flap transfer.
The donor site generally does not require angiographic clarification. Doppler ultrasound here should be sufficient.
During the first 24 postoperative hours the perfusion of the flap anastamosis is especially vulnerable. Therefore, intensive monitoring is recommended for this period at least. The required heparin administration for thrombosis prophylaxis is 150 IU heparin/kg/BW in 24 hours for 2–5 days.
Thereafter a low-molecular heparin is administered once daily until complete recovery and mobilization.
NOTE
The required heparin administration for thrombosis prophylaxis is 150 IU heparin/kg/BW in 24 hours for 2-5 days.
Special Section: Covering a Defect
Skull
Simple defects in the scalp can be covered with split-skin or full-thickness grafts. Bone defects with loss of the periosteum require a flap (Figs. 6.5, 6.6, 6.7, 6.8, 6.9).
The trabecular bone of the diploe, in contrast to the poorly perfused bone near the periosteum, can be covered with a fine skin graft. However, a local flap is preferred from a cosmetic point of view.
Defects in the face and neck can usually be covered with local flaps or pedicled myocutaneous flaps, like a pectoralis major flap.
NOTE
A skin graft is not sufficient to cover an exposed bone unless the outer layer of bone is removed and the diploe is exposed.
Extensive defects of the skull cap with necrotic portions of bone require coverage with microsurgical tissue grafting. Transplantation of a free, microsurgically anastomosed latissimus dorsi flap fulfills the requirements for sufficient soft-tissue coverage when infection exists.
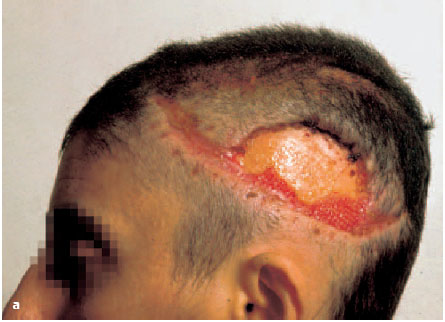
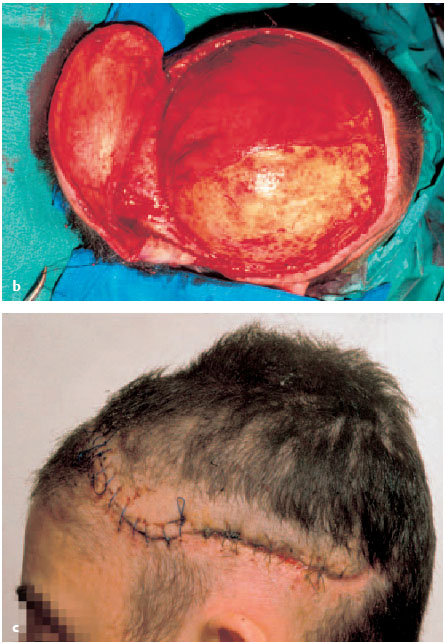
Fig. 6.5a-c Scalping and contusion injury left parietotemporal.
a After adaptation of the wound edges a necrotic zone measuring 8 × 4.5 cm appears, which is then removed. Ten weeks later the tabula externa is exposed leaving a defect measuring 6.2 × 3.8 cm. There is only mild formation of granulation tissue on the sides.
b To prevent osteomyelitis of the top of the skull, the wound edges are debrided and the tabula externa removed tangentially with a Lexer chisel untilpetechial bleeding appears. A Contralateral pedicled temporoparietal scalp rotation flap is raised and covers the defect without any tension.
c Defect coverage 1 week after the operation.
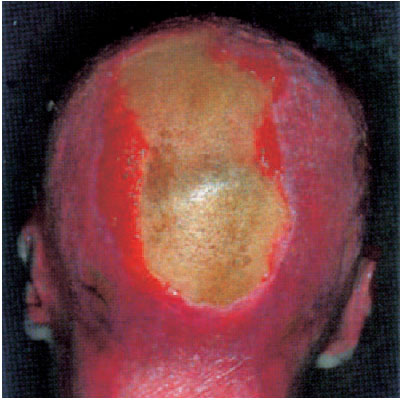
Fig. 6.6 Burns on the head and other regions. Seven months after the trauma a 22 × 8 cm defect remains with a yellowish tabula externa. The entire occipital and skullcap region has only been thinly covered with a split-skin graft.
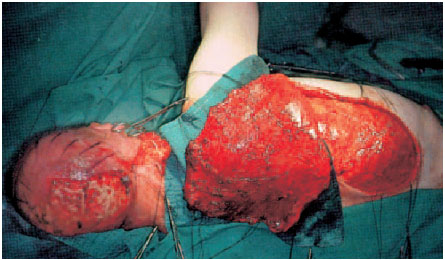
Fig. 6.7 Radical debridement with abrasion of the superficial bone and multiple trepanations in the skull until petechial bleeding appears. A latissimus dorsi flap has been raised.
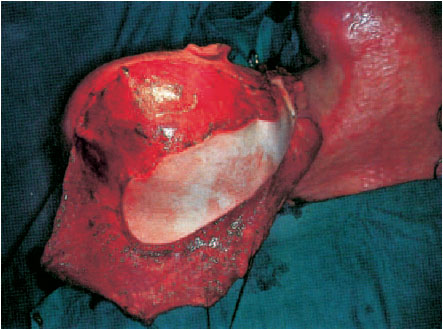
Fig. 6.8 Coverage of the skullcap and the occiput with a partial myocutaneous latissimus dorsi flap measuring 32 × 36 cm. Revascularization is achieved with an end-to-end anastamosis of the right superior thyroid artery and the external jugular vein (for the technique see Latissimus Dorsi Flap, p. 203).
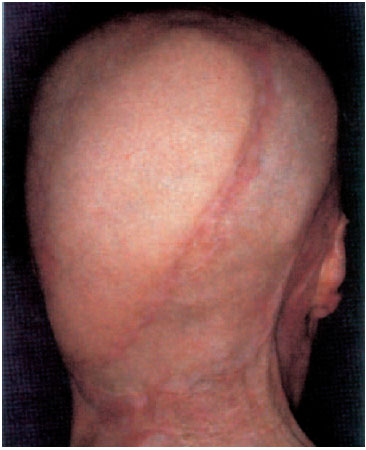
Fig. 6.9 Final result 4 years later. The skin over the occiput is normal with good soft-tissue coverage, over which a wig can be comfortably worn.
Shoulder Girdle and Upper Arm
In septic bone surgery, the pectoralis major and the latissimus dorsi muscles, as well as scapular and parascapular flaps, are primarily used to cover defects in this area.
NOTE
The maximal available flap size in adults is 32×12 cm. Since the sternocostal and abdominal edges of the muscle are generally left in place, the remaining available flap measures approximately 28 × 10 cm.
Pectoralis Major Flap
In principle, the whole muscle with adjacent skin and soft tissues can be raised as a flap due to the intramuscular branches. Numerous anatomical norm variants exist. In 30% there is no pars abdominalis, and a smooth transition to the deltoid muscle (no deltoideopectoral sulcus) often exists.
Indications:
• Defects in the lateral head/neck
• Defects in the mouth
• Defects in the thoracic wall/shoulder
Vascular and nervous supply:
Thoraco-acromial artery and vein:
• The blood vessels cross the clavipectoral fascia with one to two large branches and the accompanying nerves and proceed on the lower surface of the muscle in a mediocaudal direction. The trunks divide into several lateral branches.
• The muscle and the skin above it is reached from medial by terminal branches of the anterior intercostal arteries II-V from the region fed by the internal thoracic artery.
• Venous drainage occurs through several veins running parallel to the arteries.
• The motor innervation of the myocutaneous pectoral flap derives from the medial (C5–C7) and lateral (C8–T1) pectoral nerves.
The length of the neurovascular pedicle depends on the distribution of vessels and nerves on the lower surface of the muscle. In 40% of cases there is only one neurovascular pedicle. In the remaining 60% there is a further pedicle to the pars clavicularis or abdominalis.
NOTE
The normal length of a neurovascular pedicle is 4–8 cm.
The main neurovascular pedicle proceeds in a cranial direction and is prevented from kinking by the clavicle. The vascular course should prevent twisting of the pedicle in flaps rotated in a ventrocranial direction. This portion of the muscle receives very little additional blood supply from segmental skin vessels (Figs. 6.10, 6.11, 6.12, 6.13).
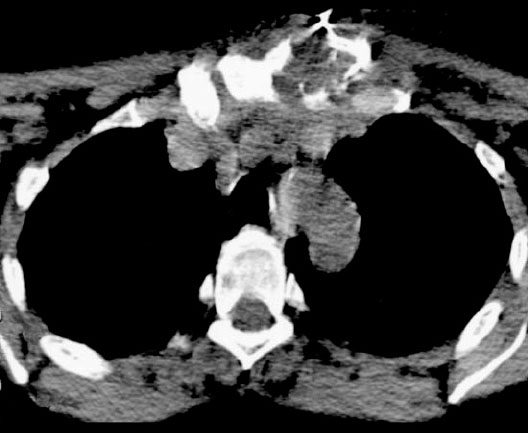
Fig. 6.10 Empyema of the left sternoclavicular joint with osteomyelitis after a cortisone injection.
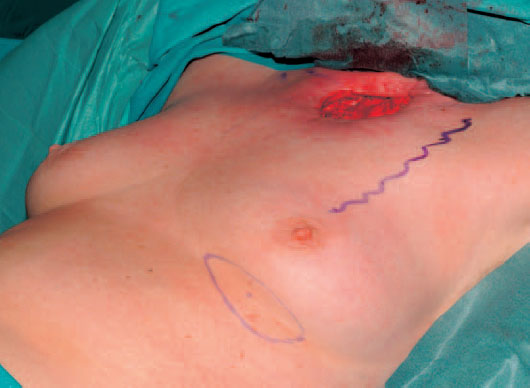
Fig. 6.11 Radical debridement with partial resection of the sternum, clavicle, and the first rib resulting in a defect measuring 8×10 cm. The pleura and mediastinum are exposed at the donor site. The course of the thoracoacromial artery and vein has been drawn.
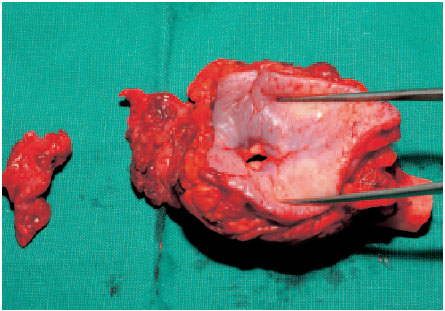
Fig. 6.12 The joint and the first rib have been resected en bloc. The fistulous sinus is shown.
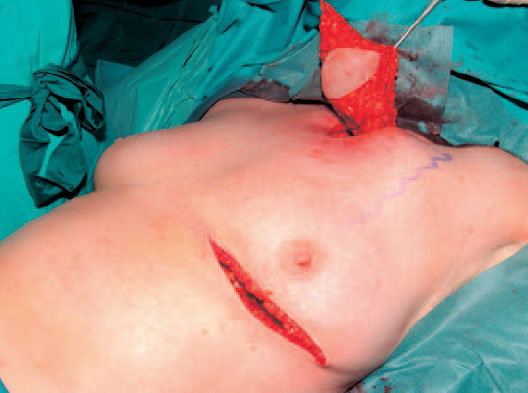
Fig. 6.13 Coverage of the defect with an ipsilateral pectoralis major island flap. The skin island is raised from the fold under the breast. After removal of mammary-gland tissue the muscle is removed from the pectoralis minor muscle beneath it and dissected from caudad to craniad. Following subcutaneous tunneling the pedicled myocutaneous graft is placed in the defect.
Parascapular Flap
Although flaps from the scapular region have no sensory nerves, this is an ideal donor region because the blood supply is reliable and the skin is thick and resistant to trauma.
A parascapular flap and a variation thereof (scapular flap) can be used either as a free flap or a pedicle flap (Fig. 6.14).
Indications:
• A pedicle flap is used to cover defects in the armpit.
• A free flap is especially suited to cover areas requiring a hearty skin graft (foot, distal portion of the lower leg).
NOTE
Complications at the donor site are few; scar tissue in the fold under the breast is generally not visible.
If a large defect requires covering, it may be necessary to cover the donor region with a split-skin graft.
If it is necessary to mobilize the flap, the muscle attachment on both the humerus and the clavicle can be severed. After dissection of the afferent vessels to their origins, a flap can be harvested to cover defects in the lower face and inside the mouth.
NOTE
Combined flaps can be obtained from the scapular region; a combination of parascapular, scapular, and latissimus flaps enable coverage of very large defects.
Vascular Supply:
These flaps contain a main branch of the circumflex scapular artery (see also Fig. 6.39). This originates from the subscapular artery and proceeds between the teres major and teres minor muscles. The subscapular artery divides into two branches. Besides the branch described above, there is the thoracodorsal artery, which supplies the latissimus dorsi muscle (see Latissimus Dorsi Flap, p. 203) on the lateral margin of the scapula. The thoracodorsal artery branches here into the parascapular artery, which runs in a caudad direction, and the scapular artery, which runs in a medial direction.
A small branch also runs in a caudad direction.
The arteries are accompanied by veins. The flaps can have a separate pedicle for each artery, or both arteries can be in one pedicle.
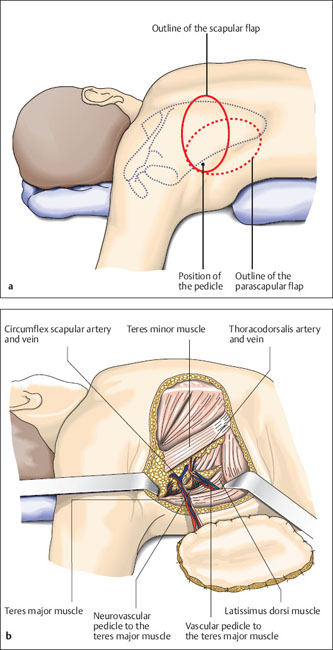
Fig. 6.14a, b Planning a flap
a The skin island is elliptical. The possibility of primary closure of the donor site after harvesting the graft can be tested preoperatively by pinching the folds of skin together in an axial direction. Dissection should always begin from medial with visualization of the vascular pedicle, because this is the safer method. Alternatively, the flap can be completely incised and dissected from the periphery to the center until the vascular pedicle is visible. Only choose this method if it is possible to precisely evaluate the relationship between the medial armpit and the top of the scapula.
b The flap is principally raised in the epifascial layer. Some fascia should also be raised around the vascular pedicle, because the first branching off of the vessels could lie just below the fascia. Tie off lateral branches of the circumflex scapular artery. Further anterior dissection of the pedicle is easy.
NOTE
The parascapular artery is larger and anatomically more consistent than the scapular artery.
Elbow and Forearm
Large, infected defects with exposed bones in the elbow region are best covered with a radial flap with a proximal pedicle. Defects in the forearm can be closed with a flap from the groin. Defects in the distal forearm, wrist, and hand can be covered well with a radial flap with a distal pedicle.
NOTE
There is retrograde venous flow through the comitans veins and the superficial venous network of the forearm. If an island flap with a distal pedicle is used, it is not necessary (but advantageous) to include a superficial vein.
Radialis Flap
A radial (forearm) flap is a fasciocutaneous flap that is harvested from the anterior aspect of the forearm. It can be transplanted as a free or a pedicled flap. Its versatility is due to the fact that it can have either a proximal or a distal pedicle (retrograde arterial flow).
Indications:
• Proximal pedicle: defects in the elbow region
• Distal pedicle: defects in the wrist, hand, and finger
• Free flap: bridging vascular defects, defects on the heel and distal tibia
Rare indications:
A free flap incorporating bones from the radius is rarely indicated.
NOTE
Before removing this flap, an Allen test must confirm sufficient perfusion of the hand through the ulnar artery. This test should be confirmed intraoperatively by clamping off the radial artery.
Vascular and nervous supply:
• Branches of the radial artery penetrate the antebrachial fascia.
• Sensory innervation is provided by the lateral antebrachial cutaneous nerve. Discriminatory sensation is, however, limited so that a free forearm flap cannot be considered a true neurovascular flap.
ERRORS AND RISKS
Disturbances in the perfusion of the thumb have been reported in rare cases. These require reconstruction of the radial artery with a venous imposition graft.
The flap limits are the edge of the ulna on the medial aspect and the middle line of the posterior forearm, including the palmar skin.
Radial Flap with a Distal Pedicle
See Figs. 6.15, 6.16, 6.17, 6.18, 6.19.
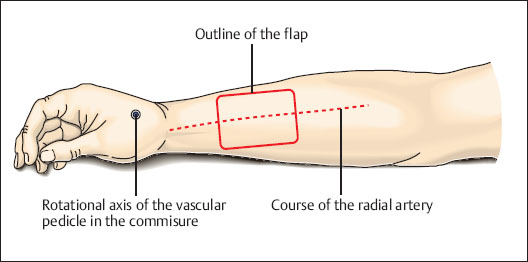
Fig. 6.15 Drawing the planned flap over the middle third of the anterior forearm. Planning the length of the pedicle and determining the rotational axis. For a defect on the back of the hand, the rotational axis is at the base of the dorsal interosseus muscle (immediately proximal of the point where the radial artery branches off). For a long pedicle, the rotational axis is at the tip of the first commissure. The deep branch of the radial artery is included in the pedicle, which is tunneled under the tendons of the thumb.
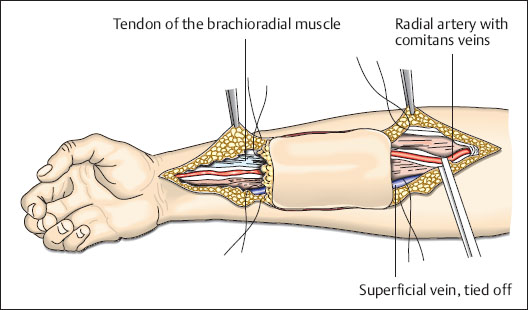
Fig. 6.16 Dissection of the pedicle with a distal incision along the radial artery. Superficial veins which enter the pedicle are ligated. Dissection begins on the ulnar side and continues to the lateral edge of the flexor carpi radialis muscle and includes the fascia. The muscle is held on the ulnar side and dissection proceeds in the deep layers of tissue. The flap is now separated from the superficial branch of the radial nerve on the radial aspect. Deep dissection is performed on the medial edge of the brachioradial muscle until reaching beneath the radial artery.
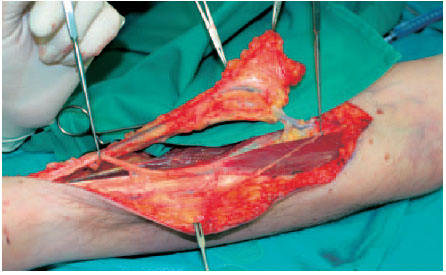
Fig. 6.17 After ligating the proximal blood vessels, the flap can be separated from the flexor muscles below it and mobilized.
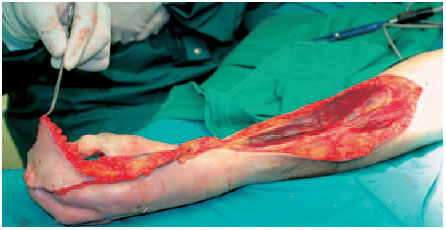
Fig. 6.18 The flap is fitted into the defect. The example shows the possible rotational arch, which reaches up to the fingertips.
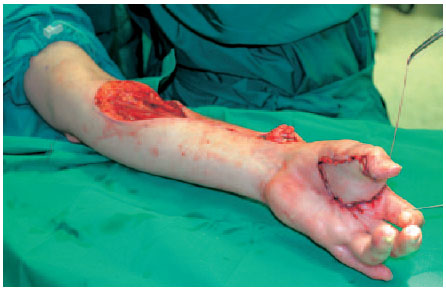
Fig. 6.19 The final steps involve covering the donor site with a split-skin graft and tunneling the pedicle subcutaneously.
Radial Flap with a Proximal Pedicle
The surgical technique corresponds to that of flaps with distal pedicles. The flap is marked out over the distal third of the forearm. A short distal incision is made to check the position of the radial artery. From proximal the pedicle can be dissected up to its origin from the brachial artery. See Figs. 6.20, 6.21, 6.22.
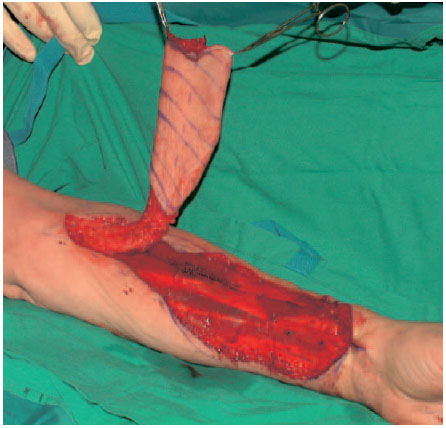
Fig. 6.20 Cutting out the radial flap in the anterior forearm with proximal dissection of the vessels. The fasciocutaneous flap is raised on the vascular pedicle.
Groin Flap
Groin flaps are among the most frequently used grafts for reconstructive surgery. Large defects, especially on the upper extremities, can be covered well with groin grafts with vascular pedicles. Because of the high variability in the diameter of its blood vessels, a groin flap is rarely used as a free flap.
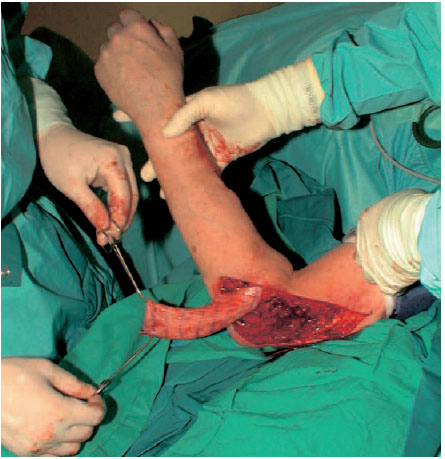
Fig. 6.21 Tunneling the skin and pulling through into the defect.
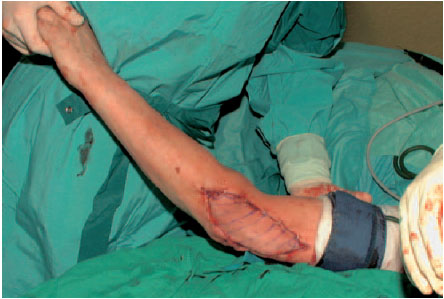
Fig. 6.22 Fitting the flap into the defect. Covering the donor site with a split-skin graft.
NOTE
Identifying the course of the artery is facilitated by orientation aids. Doppler ultrasound can help localize the site where the artery penetrates the medial edge of the sartorius muscle.
< div class='tao-gold-member'>









