Periprosthetic Joint Infection: Diagnosis and Overview of Treatment
Robert E. Mayle
Darren R. Plummer
Craig J. Della Valle
Case Presentation
A 63-year-old female presents with several months of right-sided anterolateral hip and posterior buttock pain with radiation down the anterior aspect of the thigh to the knee following a right total hip arthroplasty (THA) done several years earlier. On physical examination, she had diffuse tenderness to palpation around the right anterolateral region of the hip, and pain with limited passive range of motion of the hip. The prior incision is healed, with no appreciable erythema, fluctuance, or drainage.
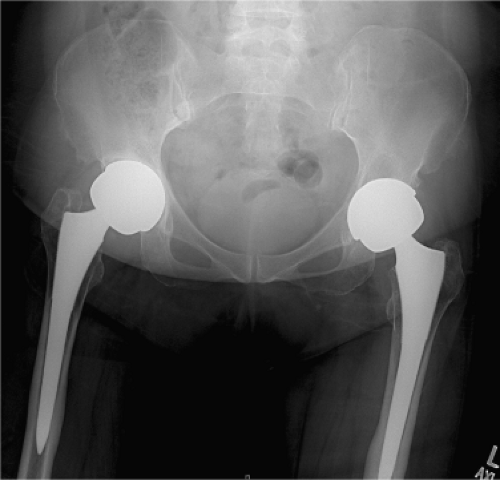
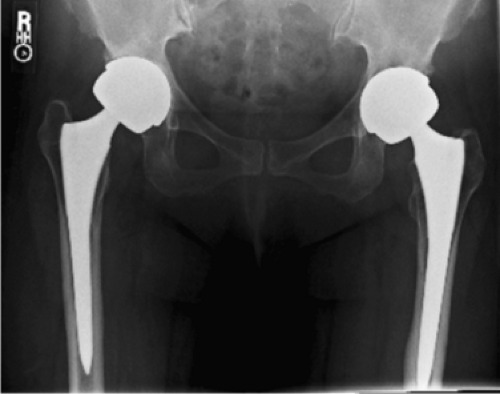
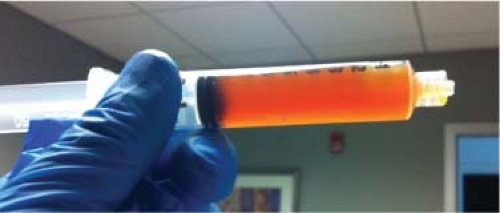
An erythrocyte sedimentation rate (ESR) was 34 mm/hr (normal 0 to 30) and a C-reactive protein (CRP) was 124.8 mg/L (normal 0 to 8.0), respectively. Radiographs of the hip were obtained (Fig. 93.1) and compared to prior radiographs (Fig. 93.2). Pertinent findings on radiograph include a radiolucent line around the acetabular component. Given the elevated ESR and CRP, a hip aspiration was performed with an 18-gauge spinal needle. Fluid obtained was seropurulent (Fig. 93.3) and was sent for culture and synovial white blood cell (WBC) with differential. In addition, fluid was placed onto a urinalysis strip to evaluate for the presence of leukocyte esterase (Fig. 93.4). Synovial WBC was 22,000 with a 95% differential. This confirmed our diagnosis of a periprosthetic joint infection (PJI).
Diagnosis of Periprosthetic Joint Infection
PJI of a THA is a devastating complication that can occur at any point in time following the index procedure. According
to the healthcare cost utilization project nationwide inpatient sample (HCUPNIS), it is the third most common indication for revision THA and was the most common reason for removal of the prosthesis (1). Medicare data from the Nationwide Inpatient Sample (NIS) estimates the incidence of infection after primary THA to be less than 1% (2). Yet, as the number of total hip replacements is expected to exponentially increase in the upcoming years, so too is the incidence of infection. In 2001, the NIS identified 4,545 patients treated for PJI after THA. By the end of 2020, the projected number of PJI cases is 16,854, an increase of 370% (3). The economic burden, as well as the functional and physical morbidity associated with PJI after THA, is overwhelming.
to the healthcare cost utilization project nationwide inpatient sample (HCUPNIS), it is the third most common indication for revision THA and was the most common reason for removal of the prosthesis (1). Medicare data from the Nationwide Inpatient Sample (NIS) estimates the incidence of infection after primary THA to be less than 1% (2). Yet, as the number of total hip replacements is expected to exponentially increase in the upcoming years, so too is the incidence of infection. In 2001, the NIS identified 4,545 patients treated for PJI after THA. By the end of 2020, the projected number of PJI cases is 16,854, an increase of 370% (3). The economic burden, as well as the functional and physical morbidity associated with PJI after THA, is overwhelming.
Accurate and timely diagnosis of PJI remains a challenging and often uncertain endeavor. Clinicians are faced with different reference criteria used to define PJI, ultimately impacting the management of patients with suspected PJI. Although no universally accepted criterion to define PJI is available, the Musculoskeletal Infection Society (MSIS) definition has recently been embraced by most orthopedic surgeons (Table 93.1). While numerous testing modalities are available to clinicians, there remains no gold standard to test for PJI. The AAOS has published evidence-based clinical practice guidelines to assist the clinician in choosing among many diagnostic tests available including a suggested algorithm for diagnosis.
Diagnosis begins by stratifying patients based on their probability of PJI. Considering a patient’s risk factors (Table 93.2), clinical presentation, and physical examination findings, patients are categorized as either high or low probability of PJI. This early determination of probability helps to guide subsequent testing; patients determined to be at high risk warrant a more aggressive evaluation whereas a less aggressive approach is appropriate for patients at lower risk.
 Figure 93.4. Fluid placed onto urinalysis strip (center box) to test for the presence of leukocyte esterase. The purple color change indicates a positive result. |
Table 93.1 Musculoskeletal Infection Society (MSIS) Definition of PJI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Well-established risk factors for PJI following THA include obesity (4,5,6), a history of superficial surgical site infection (7,8,9), and operative time greater than 2.5 hours (10,11,12). In a review of 8,494 total joint arthroplasties, patients with a body mass index (BMI) greater than 50 kg/m (2) had an odds ratio of infection of 23.1 over patients with a normal BMI (6). In addition, a number of risk factors, supported by expert consensus, should also be considered when determining a patient’s overall risk of developing a PJI (Table 93.2) (13). Although not unequivocally supported by evidence, medical comorbidities, including diabetes, renal disease, and heart disease along with behaviors such as smoking and excess alcohol consumption have all been
identified as potential factors that may increase the risk of developing a PJI (6,8,14,15).
identified as potential factors that may increase the risk of developing a PJI (6,8,14,15).
Table 93.2 American Academy of Orthopaedic Surgeons (AAOS) Risk Factors for Periprosthetic Joint Infection | ||||
|---|---|---|---|---|
|
Table 93.3 Diagnostic Evaluation of Serum ESR (Erythrocyte Sedimentation Rate) for Detection of Chronic PJI | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Serum Laboratory Tests
Laboratory tests, including an ESR and CRP, are well-accepted initial screening tests for PJI and should be obtained in all patients presenting with a painful joint or planned revision THA. These tests are inexpensive, easily performed, and readily available to most clinicians. In addition, these inflammatory markers have proven to be highly sensitive for detecting PJI (16,17,18,19,20,21). Both ESR and CRP offer high diagnostic value as individual tests, with reported sensitivities of 94.5% and 94.3%, respectively; when the tests are combined, the sensitivities increase to as high as 97.6% for ruling in or out PJI (20).
Furthermore, clinical series shows that the ESR and CRP are rarely both normal in the face of PJI. In a study of 235 consecutive revision THAs, Schinsky et al. (17) reported that no infections were found in patients with normal ESR and CRP. Similar results were found by Spangehl et al. (22), concluding that the combination of a normal ESR and CRP are reliable and highly predictive of the absence of infection. Inflammatory arthritis has commonly been assumed to render the results of ESR and CRP inaccurate and unreliable. However, in a study of 871 total joint arthroplasties, 61 with inflammatory arthritis and 810 without, ESR and CRP were found to be effective for diagnosing PJI in patients with inflammatory arthritis as well as noninflammatory arthritis with similar optimum cutoff values and overall testing performance (Tables 93.3 and 93.4) (23).
In summary, the ESR and CRP are excellent screening tests for PJI. These screening tests are strongly recommended by the AAOS clinical practice guidelines (25) and should be obtained in all patients suspected of PJI and prior to any revision total joint arthroplasty.
Synovial Fluid Analysis
Aspiration of synovial fluid is considered by many to yield the highest diagnostic value in the workup to identify PJI (25). Hip aspiration typically requires the use of ultrasound or fluoroscopic guidance, which increases procedural time, expense, and can limit practical application to many clinicians. Because synovial fluid aspiration of the hip is more painful, poses greater potential risk to the patient, and is more technically challenging to perform, a more selective approach is recommended than aspiration of the knee. In a patient with suspected PJI of the hip, if both ESR and CRP are elevated, a joint aspiration should be performed (25). If only one of these inflammatory markers is elevated, the physician’s judgment and clinical suspicion should guide the decision to perform an aspiration. If clinical suspicion is high for PJI based on patient specific risk factors and presentation, a joint aspiration should be performed. Conversely, if the clinical suspicion is low, reoperation status should be considered. If revision surgery is planned, an intraoperative joint aspiration may be performed or tissue specimens obtained for an intraoperative frozen section. If revision surgery is not planned, the patient should be reevaluated within 3 months. Finally, if both ESR and CRP are within normal reference ranges, joint aspiration is only recommended if suspicion of PJI is high.
Once synovial fluid is obtained, analysis of the fluid WBC count and percentage of neutrophils, along with aerobic and anaerobic cultures, should be obtained. Analysis of synovial
WBC count and neutrophil percentage has gained acceptance as possibly the best diagnostic test to differentiate PJI from aseptic failure. While the diagnostic accuracy of synovial fluid WBC count and neutrophil percentage are well supported by multiple studies with respect to knee aspirations, limited studies have been conducted specific to hip aspirations.
WBC count and neutrophil percentage has gained acceptance as possibly the best diagnostic test to differentiate PJI from aseptic failure. While the diagnostic accuracy of synovial fluid WBC count and neutrophil percentage are well supported by multiple studies with respect to knee aspirations, limited studies have been conducted specific to hip aspirations.
Table 93.4 Diagnostic Evaluation of Serum CRP (C-Reactive Protein) for Detection of Chronic PJI | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|