Fig. 7.1
Type A pelvic ring injuries. The pelvic ring is mechanically stable
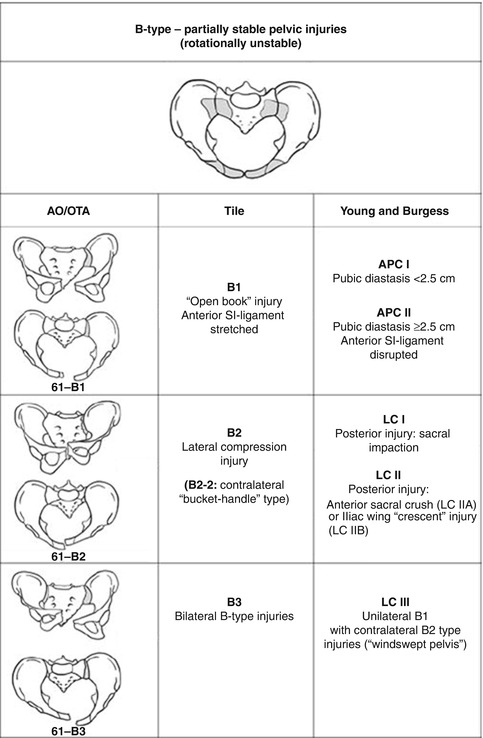
Fig. 7.2
Type B pelvic ring injuries. The pelvic ring is rotationally unstable but the posterior SI ligaments are intact
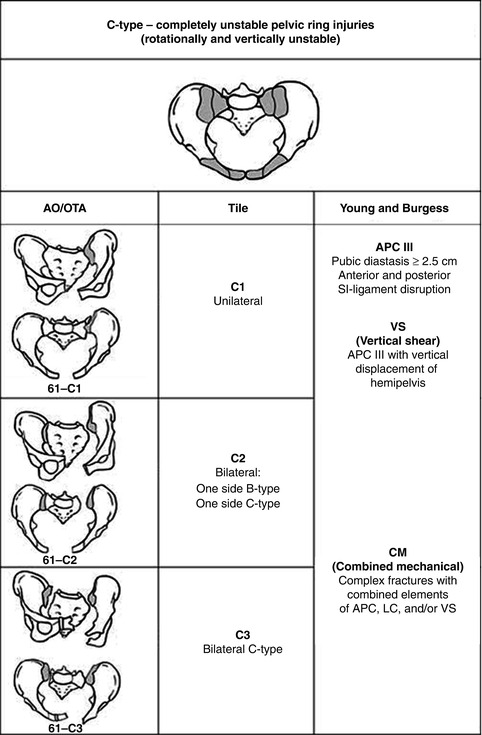
Fig. 7.3
Type C pelvic ring injuries. Rotationally and vertically unstable pelvic ring injuries with complete disruption of both anterior and posterior SI ligaments
Young and Burgess (Mechanism of Injury Classification)
The Young and Burgess classification is based on the force vector of injury to the pelvic ring. There are three types of injury patterns: (1) anterior/posterior compression (APC), (2) lateral compression (LC), and (3) vertical shear (VS).
The APC-type injuries are divided into three types. Type I injuries are due to an anteroposterior force that “opens” the pelvis like a book with intact posterior ligamentous structures. Type II injuries are a type I injury with disrupted sacrospinous +/− sacrotuberous ligaments as well as the anterior SI ligaments. A type III injury is complete disruption of all ligaments (“open book”) and associated with retroperitoneal rather than intraperitoneal hemorrhage. APC types II/III are unstable.
The LC-type injury is divided into three types as shown. Type I caused by a posteriorly directed force resulting in a sacral crush injury with ipsilateral horizontal pubic rami fractures. Type II is a more anterior-directed force with a resulting anterior sacral crush and ipsilateral rami fractures and injury either to the ilium (i.e., “crescent” fracture) or SI joint. These injuries have a high incidence of associated head and/or intra-abdominal injuries. Type III injury is more severe than types I/II due to an external rotation component to the contralateral side and possible internal rotation component to the ipsilateral side (so-called windswept pelvis). This injury results in a similar fracture pattern to an LC II injury with additional disruption of the sacrospinous and sacrotuberous ligaments. As discussed before, due to injury to the posterior SI ligaments and sacrotuberous and sacrospinous ligaments, the pelvis is rotationally, vertically, and posteriorly unstable. Thus, an LC III injury is a mechanically unstable pelvis injury due to a more anterior, lateral-directed force.
Comprehensive Pelvic Disruption Classification (Modified After Tile)
This classification scheme is based on the mechanism of injury as well as the degree of pelvic stability. This system is based on whether the posterior arch of the pelvis is disrupted and is summarized per Figs. 7.1, 7.2, and 7.3. Type A injuries do not affect the mechanical integrity of the pelvic ring (avulsion of the innominate bone, iliac wing fracture, etc.). Type B injuries are rotationally unstable with partially stability of the posterior pelvic ring, while type C injuries are completely unstable anterior and posterior pelvic ring injuries. Figure 7.4 demonstrates the correlations between the Young and Burgess and Tile classification patterns.
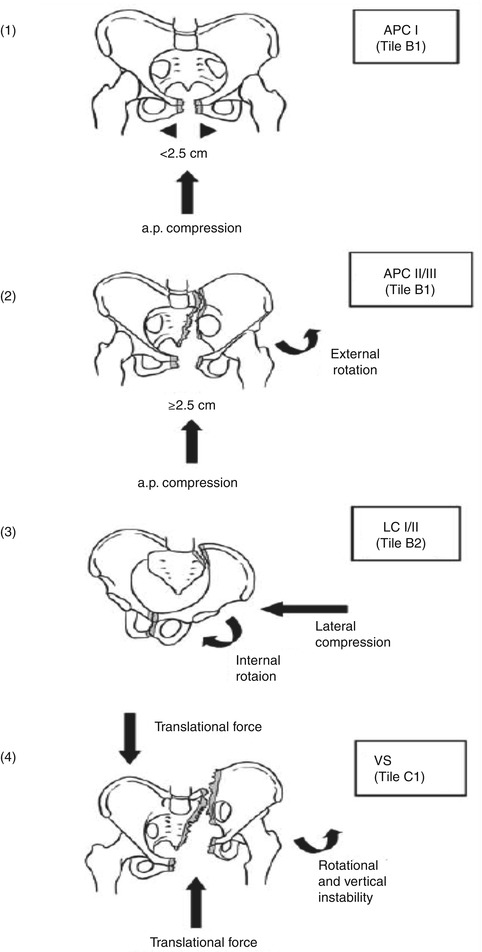

Fig. 7.4
A correlation between the Young/Burgess and Tile classification schemes
AO/OTA Classification
This classification is similar to the Tile classification in that it is based on the stability of the posterior pelvic structures. Type A fractures are stable fractures with no involvement of the mechanical ring (50–70 % incidence). Type B fractures are partially unstable injuries with partial posterior, rotational instability after anteroposterior or lateral compression (incidence 20–30 %). Type C fractures are unstable injuries with combined anterior and posterior vertical instability (incidence 10–20 %).
Although these classification schemes are useful in deciding if treatment is necessary, it is important to realize that in many cases, patients will present with a combined mechanism (CM) fracture pattern. In these cases, the surgeon should base his/her decision on the patient’s clinical scenario and basic biomechanical principles. While fracture classification is important in identifying mechanically and potentially hemodynamically unstable pelvis fractures, minimal time should be spent deciding upon classification during the initial evaluation. Obtaining extra X-rays, special views, or CT scans in order to classify the fracture has no place in the acute setting of the unstable or borderline stable patient.
Assessment of Pelvis Fractures
History
In assessing pelvic injuries, the patient must be fully evaluated including history, physical exam, and appropriate radiographic studies. If the patient sustained a high-energy injury or is hemodynamically unstable, the ATLS protocol should be followed. This assessment is important since both the history and physical examination offer clues to fracture severity and potentially guide treatment. For example, a fall from a low height or ground level in an elderly individual may imply a stable fracture type, whereas a high-energy injury, i.e., motor-vehicle accident or fall from a large height, would suggest an unstable injury pattern. Nevertheless, it is important to take the entire patient’s clinical scenario into consideration, since a seemingly low-energy pattern in an elderly individual may cause significant morbidity and mortality.
As mentioned before, high-energy injuries to the pelvis are associated with significant mortality due to hemorrhage. Associated injuries include urogenital injuries in 10–15 % of patients [34] with the bladder and the male urethra the most common sites of injury [35]. Sexual dysfunction is also a common, and often underreported, complication of pelvic fractures with an average rate of 35.9 and 39.6 % in men and women, respectively [36]. Lumbosacral plexopathy injury is present in 10–15 % of patients [37] with pelvic fractures. Risk factors for neurologic deficits include unstable pelvic and/or sacral fractures with the L5 and S1 nerve roots most at risk [38]. In addition to the mortality rates as discussed before, 60–80 % of these patients have associated musculoskeletal injuries.
Physical Examination
Physical examination should follow the ATLS principles of A (airway), B (breathing), C (circulation), D (disability), and E (extremity). In some cases, pelvic injuries may have dramatic presentations including open fractures and gross deformity. The evaluating trauma team must remain disciplined, however, and not skip initial evaluation steps—i.e., airway is more important than X-ray. Critical aspects of the standard ATLS workup of these patients include identifying the sources of hemorrhage. Part of the primary survey entails inspection for ecchymosis or compromised skin around the pelvis or genitalia. Blood at the urethral meatus, scrotal hematoma (Destot sign), and/or a high-riding prostate on rectal exam indicate pelvic disruption with possible bladder injury. Signs of pelvic instability include leg-length discrepancy with either shortening or external rotation on the involved side. The posterior aspect of the pelvis should be examined for palpable defects along the sacroiliac joint, hematoma, or ecchymosis. Studies have shown that palpation of the posterior pelvis in patients with pelvic fractures can accurately detect injuries of the posterior ring in the awake patient [39]. The absence of posterior sacral tenderness in a cooperative, alert patient can nearly rule out a posterior pelvic injury [40]. On the contrary, the presence of pelvic deformity or an unstable pelvic ring on physical examination has poor sensitivity for diagnosing a mechanically unstable pelvis in blunt trauma patients [40]. In awake, alert, and cooperative patients, a standard neurologic exam should be performed to assess the lumbosacral plexus since there can be significant neurologic damage, especially if the posterior injury is a sacral fracture [41, 42].
Radiographic Examination
According to Young and Burgess, 95 % of pelvis injuries can be diagnosed from an anterior/posterior (AP) pelvis film [8]. A standard AP pelvis radiograph along with inlet and outlet views as described by Pennal [4] should be obtained. Inlet and outlet views demonstrate injury to the pelvic ring and vertical instability of the pelvis, respectively. Finally, CT has proven to be invaluable in assessing injury to the posterior pelvic ring, and given the current technological advancements, CT can be done in an efficient and expeditious manner.
While the abovementioned studies are useful in understanding the fracture, it is important to emphasize, as mentioned earlier, that inlet/outlet views and CT scans have absolutely no role in the hemodynamically unstable pelvic injury patient, in terms of imaging the bony injuries, pelvic hematomas of fracture categorization. These studies do not in any way reduce shock or assist the surgeon in controlling blood loss. The AP pelvic X-ray, however, is critical in allowing the treatment team to decide whether the pelvis could be a source of shock. Bleeding in the pelvis can originate from damage to (1) the venous plexus anterior to either the posterior pelvic ring and/or the bladder, (2) the common/external/internal iliac arteries/veins, and/or (3) the fracture bone surfaces. If the source of hemorrhage is arterial, the most common site of injury is the internal iliac artery or a branch thereof. Huittinen and Slätis [43], in a seminal autopsy study on patients with pelvic fractures, demonstrated that in 85 % of deaths, bleeding was from bone surfaces and veins; in only 15 % of mortality cases could they identify an arterial source of bleeding. Thus, management strategies must be directed primarily at controlling bone and venous bleeding and secondarily at arterial bleeding.
Initial Management
Initial management of the patient with a pelvic injury follows ATLS guidelines.
Standard 14 or 16 gauge peripheral intravenous (IV) cannulas should be used for resuscitation. A 2-L bolus of IV crystalloid should be given to hypotensive patients. If the blood pressure or urine output does not improve, another 2 L bolus followed by O-negative (non-crossmatched) blood should be administered promptly. Type-specific blood, crossmatched to ABO and Rh type, can be administered once available (approximately 30 min at most institutions). If blood transfusion is required, fresh frozen plasma (FFP) and platelets should be given in a 1:1 ratio with packed red blood cells. The 1:1 ratio of FFP to PRBC should be started immediately [44], and platelets should be coadministered due to the demonstrated platelet dysfunction accompanying traumatic coagulopathy [45]. This 1:1 ratio of FFP-PRBC administration has demonstrated improved survival to discharge by decreasing death from hemorrhage [46]. Although there is recent controversy regarding the ratio of FFP to PRBC, the amount of crystalloid administration, the use of fibrinogen with/without FFP, and recombinant coagulation factor usage, the authors recommend a 1:1 ratio of FFP-PRBC until further prospective randomized controlled trials are done. Despite the controversy, one of the most common errors in pelvic resuscitation is delaying the transfusion of clotting factors. If the definitions and markers of hemodynamic instability are not universally understood, the patient at greatest risk for under-resuscitation is the borderline patient, characterized by transient responses to fluid or blood. In these patients, FFP is often omitted initially. Once they become hypotensive, however, they have already received significant fluid and undergo a rapid dilutional coagulopathy that is difficult to reverse and often ends in death. Therefore, once the first unit of red blood cells is transfused, the authors recommend converting to a preplanned protocol of 1:1 transfusion of RBC-FFP. This single strategy may be the most critical improvement in resuscitation that has resulted in decreased mortality. Unfortunately, it is often neglected due to the lack of standardized protocol requirements at most trauma centers.
Core body temperature should also be measured and kept as close to 37 °C as possible during initial evaluation and resuscitation since hypothermia results in impaired coagulation. If there is suspected ongoing bleeding, 1 of the 5 sources must be identified: external (on the field), thoracic cavity, fracture (long bones), abdomen (intraperitoneal), and/or pelvis (retroperitoneal). Ongoing evaluation and reevaluation for hemorrhagic sources such as the Focused Abdominal Sonography for Trauma (FAST) exam, direct peritoneal lavage (DPL), high-speed CT, and/or exploratory laparotomy should be implemented based on protocol and surgical judgment. Pelvic angiography has an important role in nonresponders with negative FAST examinations but is not recommended as a primary diagnostic tool in the hemodynamically unstable patient. In fact, Hou et al. have demonstrated, in a small cohort of trauma patients, that primary angiography can be detrimental [47].
Initial stabilization of the “open-book” or APC-injured pelvis can be done by wrapping a sheet around the pelvis and closing down the retroperitoneal volume, thus helping to tamponade ongoing bleeding. This stabilization can also be accomplished by devices such as the Trauma Pelvic Orthotic Device (T-POD®, Pyng Medical), SAM Pelvic Sling® (SAM Medical Products), a C-clamp, or external fixation (Fig. 7.5). The physician should pay careful attention to the location of the binder. Frequently, the binder placement is too high and results in an inadequate pelvic reduction. Recent literature has demonstrated that accurate placement of a pelvic binder, at the level of the greater trochanter, improves reduction of pelvic ring diastasis while permitting unobstructed access to the abdomen for laparotomy [48]. All these devices function as splints to decrease pelvic volume, stabilize the bone and soft tissues, decrease laceration of small blood vessels, protect intrapelvic clot formation, and decrease catecholamine release. In patients who are obese, internal rotation and taping of the lower extremities can be done if a pelvic binder cannot be placed [49]. Initial stabilization of VS-type injuries to the pelvis includes a traction pin, in addition to an external fixator or other device, placed in the vertically unstable pelvis to help pull the displaced hemipelvis into a more anatomic and stable position. Mechanically stable fractures and LC-type injury patterns do not require volume closure or splinting. However, patients with apparently stable fracture patterns who are taken urgently for laparotomy, thoracotomy or pelvic packing should be quickly and carefully reevaluated for mechanical instability in the operating room. A recent retrospective review of 68 patients demonstrated occult instability in 50 % of presumed APC I, 39 % of APC II, and 37 % of LC 1 pelvic injuries [50]. In some cases, stable appearing fractures are indeed unstable once stressed under anesthesia using fluoroscopy. APC-type injuries will demonstrate increased pubic diastasis after anteroposterior-directed force on the iliac crest. LC-type injuries will be exacerbated (i.e., more internal rotation or displacement) after a laterally directed force. Vertical shear injuries can be evaluated by the “push-pull” test in which longitudinal traction or compression is exerted on the affected lower extremity and displacement of the hemipelvis is assessed using dynamic fluoroscopy. For hemodynamic and/or mechanically unstable pelvic injuries assessed in the operating room, a simple form of external fixation device can be rapidly applied if the reexamination shows dynamic instability or is equivocal.
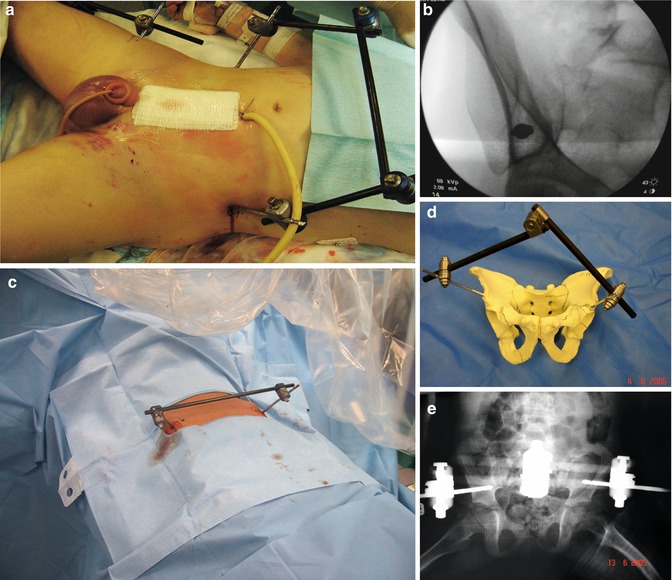
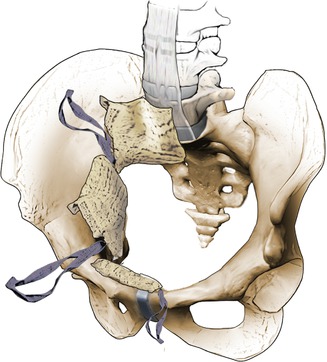

Fig. 7.5
Pelvic packing is performed after external fixation so that the surgeon can pack against a “stable” pelvis. The external fixator can be placed in the iliac crests (a) or in the anterior inferior iliac spine (AIIS)-between the two tables of the pelvis in the supra-acetabular region (b). In either case, the external fixator is placed to allow access to the abdomen and pelvis (a, c). The position of the AIIS pins is demonstrated in a bone model (d) with corresponding X-ray (e). Due to the strong bone stock in the supra-acetabular region, an anterior external fixator frame is more stable; however, fluoroscopy (b) is usually more necessary for accurate pin placement

Fig. 7.6
Schematic drawing which demonstrates placement of laparotomy sponges for pelvic packing. All sponges are placed in the true pelvis below the pelvic brim. Three sponges are placed on each side of the pelvis (anterior to the sacrum, medial to the quadrilateral plate, and in the space of Retzius) either via a Pfannenstiel incision or a longitudinal (laparotomy), midline incision (Artwork by Bernie Kida)
During the workup of these patients, if a urethral injury is suspected based on physical exam (i.e., blood at the urethral meatus or in the vagina), Foley catheterization should not be done since the catheter could potentially disrupt an already existing urethral tear. If a bladder injury is suspected and the patient is stable, a cystogram can be performed.
During the initial assessment of the patient with an unstable pelvic fracture (AO/OTA type B or C), particular attention should be paid to the patient’s age, Revised Trauma Score (RTS), and blood transfusion requirements since all have been shown to be predictors of early mortality [13, 14]. Specifically, age >60 and Revised Trauma Score (RTS) are easily accessible during the assessment and suggest the severity of the injury and risk of mortality [13, 14]. The RTS is a physiological scoring system obtained from the sum of the Glasgow Coma Score (GCS), systolic blood pressure (SBP), and respiratory rate (RR)—with lower scores associated with increased mortality. Although ISS also correlates with early mortality, it is not easily determined upon initial assessment. As discussed before, fracture pattern has not always been shown to correlate with mortality nor can it be used to determine the need for angiographic embolization [51]. Despite numerous relevant indicators of potential mortality, the three most useful to the evaluating team are age, shock, and transfusion requirements. These require no calculation and are apparent to even minimally trained staff. Furthermore, each indicator has been shown by various investigators to be a predictor of high mortality. For example, Sathy et al., in a study of 63,000 trauma patients, found the odds of mortality in patients aged over 60 years with a pelvic fracture to be 4.5–8.8 times higher than patients less than 60 [52]. The same study also demonstrated that a systolic blood pressure <90 mmHg is a key predictor of mortality in pelvic fracture patients [52]. Therefore, when one of these risk factors is present, the treatment team should immediately presume the patient is “unstable” until clearly proven otherwise. The trauma team should also obtain an initial arterial blood gas and monitor the base deficit during management to assess physiologic stability. The cause of death for unstable pelvis fracture patients in the first 24 h is most commonly due to acute blood loss. After 24 h, the cause of death is usually from multiple organ failure; thus, the cornerstone of treatment should be aggressive shock management, with the goal to decrease the required transfusion volume. Preplanned protocols involving a multidisciplinary management approach are essential and have been shown to lower mortality in these severe injuries. Therefore, if the patient is elderly, in shock, or requires a transfusion, the management team should initiate a preplanned protocol immediately. If the patient stabilizes quickly, then the protocol can be discontinued. Since the goal of most protocols is to avoid “under-triage,” some “over-triage” is expected and necessary. However, if the surgeon neglects the borderline patient with the aforementioned warning signs due to a false sense of “stability,” this is a grave error that may result in the patient’s mortality.
Mechanical and Hemodynamic Instability
In the workup of patients with pelvic fractures, two aforementioned factors must be considered since they will guide both resuscitation and treatment: (1) mechanical instability and (2) hemodynamic instability. Rommens demonstrated higher mortality in higher-energy pelvis injuries in 122 patients with unstable B- and C-type injuries [16]. Burgess et al., in a series of 210 patients, correlated fracture type with transfusion requirements: LC, APC, VS, and CM injuries required 3.6, 14.8, 9.2, and 8.5 units of PRBC, respectively [9]. Mortality from LC and APC patterns was due to closed-head injury and combined pelvic/visceral organ injury, respectively [53]. Similarly, Magnussen et al., in a retrospective review of 382 patients with isolated pelvic and/or acetabular fractures, demonstrated that APC II/III, LC III, VS, or CM pelvic injuries required more frequent transfusion than other pelvic fractures [54]. These correlations of fracture pattern and mortality should serve as a guide, but not as a definite rule, since patients can still exsanguinate from more “benign” fracture patterns. For example, Smith and Starr demonstrated that specific fracture patterns were not predictive of mortality [13, 14]. Mechanical instability needs to be determined to reduce the risk of further bleeding, decrease pain, and to allow early mobilization. Hemodynamic instability needs to be addressed by standard ATLS protocols for the obvious reasons to decrease mortality. Thus, these two issues, while not necessarily predictive, should be considered related. For example, a lateral compression type I (LC I) pelvic fracture is not an “unstable” definition but can still result in significant retroperitoneal hemorrhage (venous > arterial) in an elderly patient with osteoporotic bone. Thus, hemodynamic instability is possible with a mechanically stable fracture pattern.
Mechanical instability of the pelvis can occur in three planes: rotational, translational, and vertical. As discussed before, the sacrotuberous and posterior SI ligaments contribute to vertical and posterior stability, whereas the sacrospinous and anterior SI ligaments contribute to rotational stability. It is difficult to assess specific damage to these ligaments, but the fracture patterns can clue the surgeon to the severity of the injury and thus the treatment needed.
The basic premise in all the fracture classification schemes is the importance of the posterior ligamentous structures in the pelvic ring. Partial or complete disruption of these structures contributes to an unstable pelvic ring injury, particularly with rotation. Specifically, the LC III, AP II/III, and vertical shear injuries all have disruption of the posterior ring and thus are unstable pelvic injuries. Clear indications for surgical stabilization of the pelvic ring are rare in type A fractures. Stabilization of the anterior ring is usually sufficient for type B fractures, while anterior/posterior stabilization is necessary for type C fractures.
As mentioned before, the history (i.e., patient age, high vs. low energy, exsanguination) can aid the surgeon in determining whether the injury is an unstable pelvic fracture pattern. Similarly, the physical exam can also clue the surgeon to the instability of the fracture pattern (i.e., lack of tenderness to palpation posteriorly). However, AP/lateral compression tests are not sensitive examinations and should only be performed once to avoid disrupting a pelvic hematoma.
Management of Unstable Pelvic Fractures
Once a patient is identified as unstable or potentially unstable, a series of preplanned steps should take place. Numerous protocols have been reported in the literature, and to some degree, all show improvement in diagnosis, management, and outcome compared to no protocol. Controversy exists regarding which is the “ideal” protocol, and it is clear from the experience of high-volume trauma centers that there is no single protocol that fits all centers. The rationale for each specific interventional methodology is relatively anecdotal and institution-dependent as these studies are ongoing. As noted, however, the literature has identified the key priorities required to reduce mortality: rapid identification of bleeding sources, graded resuscitation with clotting factors, and interventions to stop bleeding, including laparotomy, pelvic packing, and angiography. As improvements in early hemorrhage, source identification and stabilization have evolved, so have resuscitative methods for patients involved in major trauma. Damage-control resuscitation (DCR), which has been passed down from the military, has continued to evolve over the past 10 years. The key components to DCR include (1) transfusion protocols with fixed ratios of blood products (PRBC, FFP, and platelets), (2) permissive hypotension to minimize hemorrhage, (3) prevention and aggressive treatment of hypothermia, (4) temporizing acidosis correction with exogenous buffer agents, and (5) use of recombinant blood products [55]. The optimal ratios of blood products as well as the type of coagulation factors have yet to be determined as more randomized controlled trials are in process. The future may entail goal-directed resuscitation as newer techniques such as point-of-care rapid thromboelastography (TEG) provide more detailed assessment of trauma-induced coagulopathy [56]. Still, despite the evolution of DCR, mechanical means of hemorrhage control via pelvic packing have demonstrated increasing efficacy at stabilizing the patient. Pelvic packing has not been widely adopted across the United States of America, and the trauma team is ultimately left with designing protocols that fit the proven pathophysiology and the resources of a given institution. The current protocol recommended by the authors has been developed over a 27-year period and represents the fourth iteration since the original paper by Moreno et al. in 1976 [57]. Each iteration was based on an evaluation of a prospective registry for pelvic trauma and was published for critique and discussion in the peer-reviewed literature [14, 46, 57, 58]. Currently, the protocol emphasizes the common pathways for all trauma patients: rapid ATLS assessment, shock control with transfusion and blood products, and simple mechanical stabilization with a binder or sheet, if indicated. If more than 2 units of RBC transfusion are required, the patient is taken immediately to the OR for direct retroperitoneal pelvic packing, C-clamp or ex-fix, laparotomy, and damage-control external fixation of extremity fractures as needed. If there is subsequent, ongoing shock or hemodynamic instability, the patient then undergoes angiography and pelvic embolization if appropriate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







