An Overview of Autologous Skin Grafts and Advanced Biologics for the Diabetic Foot
Keywords
• Diabetic neuropathy • Ulcer • Surgery • Skin grafts • Orthobiologics • Diabetic foot
Autologous STSGS for the diabetic foot
While the modern meshed skin graft was first described in 1964,1 the first documented use of skin grafts dates back to 3000 BC in India for soft tissue reconstruction of facial trauma.2 This technique has evolved through the years to provide a relatively simple, quick, and highly effective method for closure of certain foot wounds.
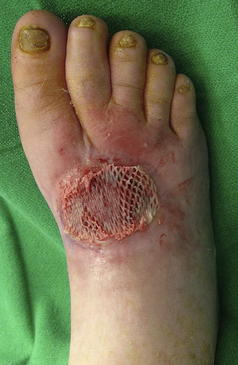
STSGs require a well-perfused granular wound bed that is free of infection typically located at non–weight-bearing aspects of the diabetic foot. STSGs involve harvesting the epidermis and a variable thickness of the upper layers of the dermis, leaving the remaining layers of dermis to heal by secondary intention.3 Donor sites may include the thigh or the ipsilateral or contralateral lower extremity of the leg and foot for smaller wounds. Once the recipient wound bed is adequately prepared after sharp manual or mechanical debridement, local hemostasis is achieved and the recipient site is accurately measured. The donor site is usually prepared by local subcutaneous infiltration of 1% lidocaine with epinephrine, and the donor skin is topically prepared with mineral oil. The most common STSG for the diabetic foot has a thickness of 0.018 in and is harvested with an electric dermatome in the length measured for the recipient site. For larger recipient sites, more STSG is harvested with additional passes of the electric dermatome. The harvested STSG is meshed in a 1:1.5 ratio using a commercially available mesher and then secured to the recipient site under minimal tension using skin staples. A bolster dressing or negative pressure wound therapy (NPWT) device can be used to firmly secure the harvested STSG in place during initial incorporation. Weight-bearing status in the postoperative period varies based on the recipient wound location and surgeon’s preference (Fig. 1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree