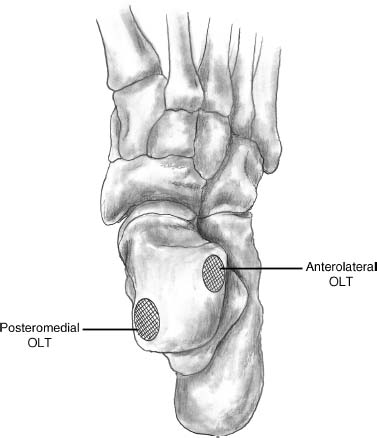
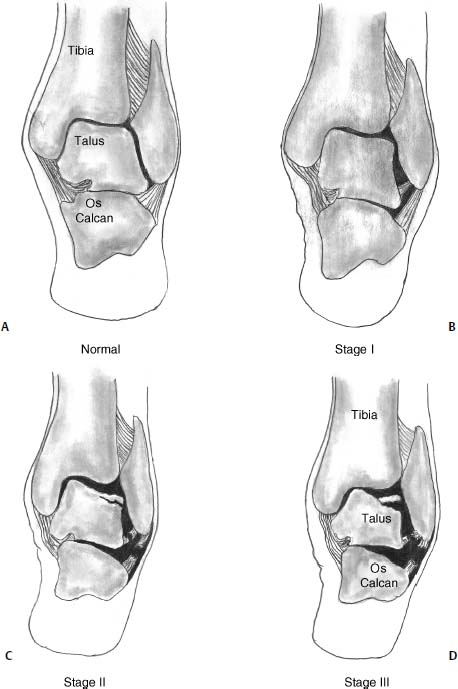
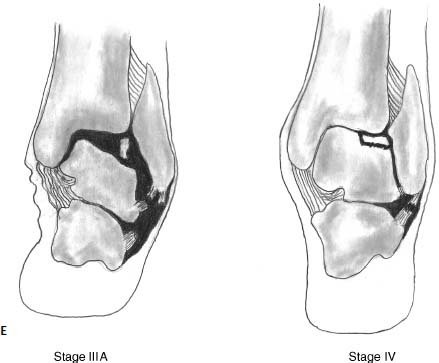
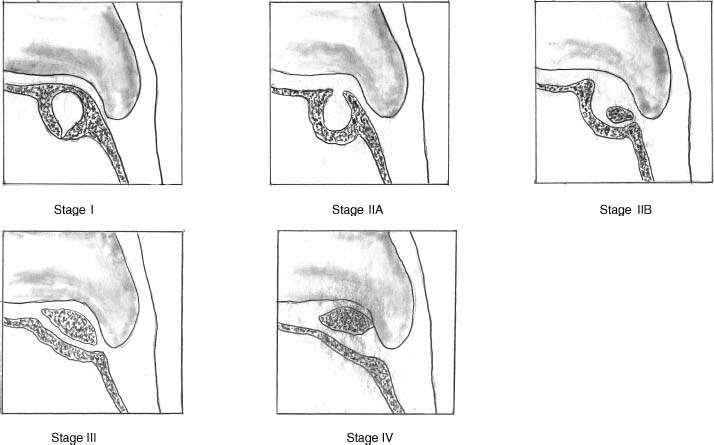
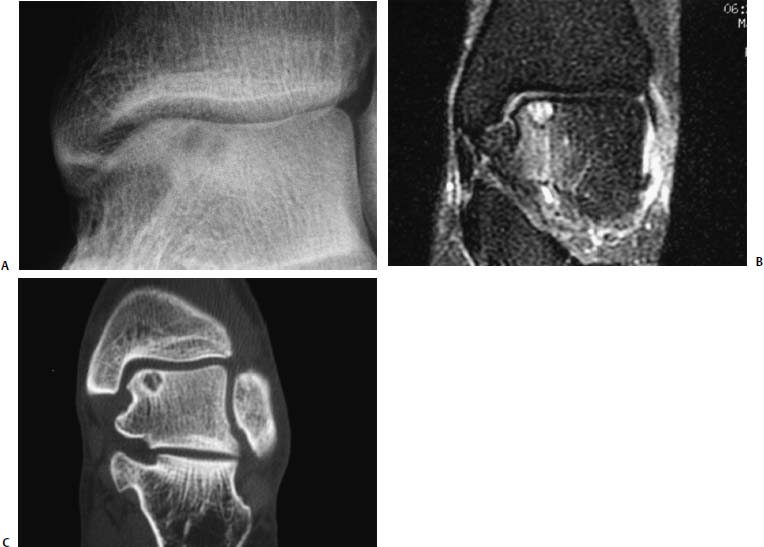
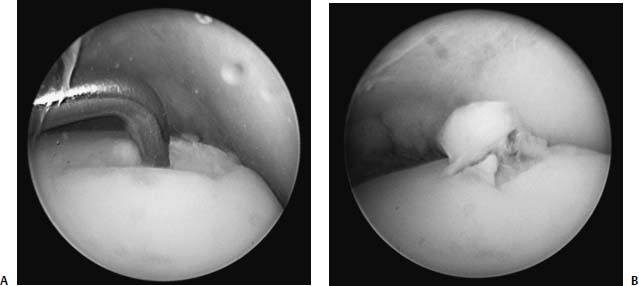
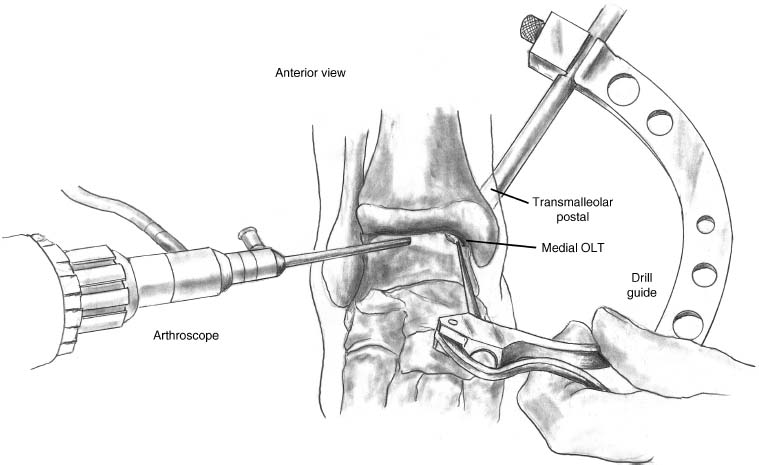
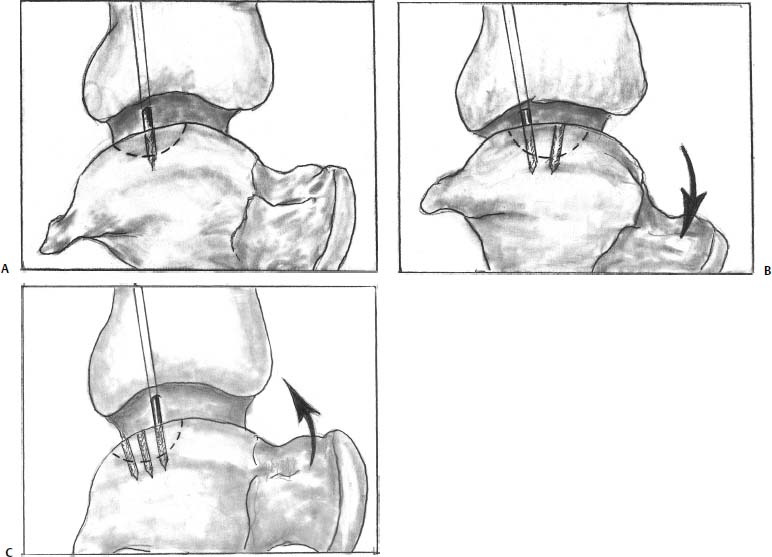
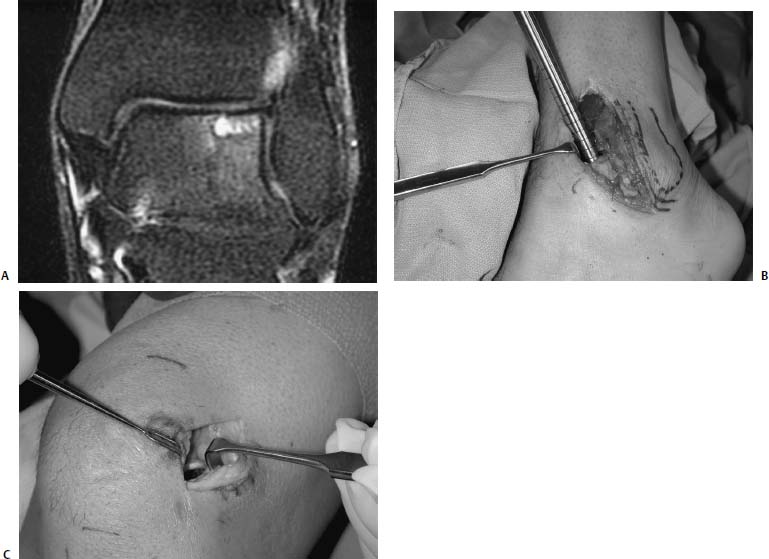
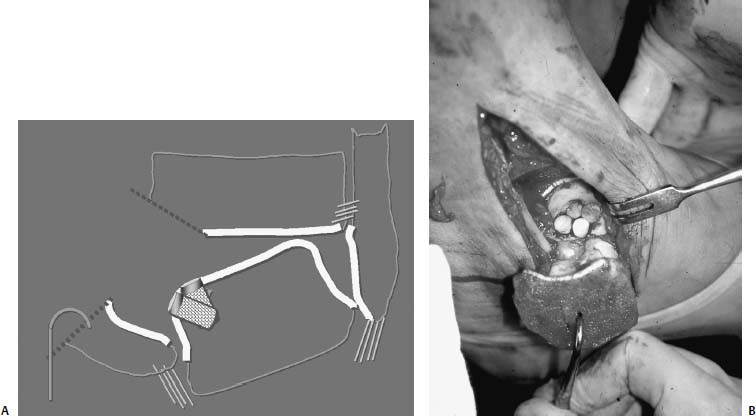
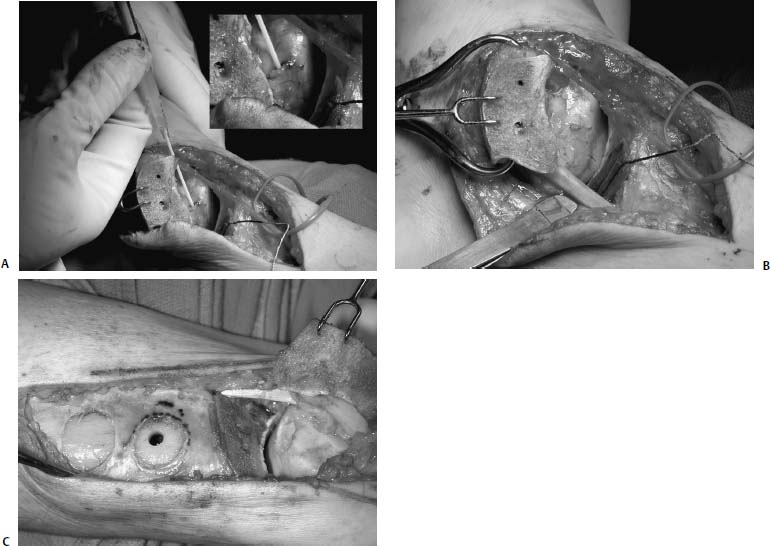
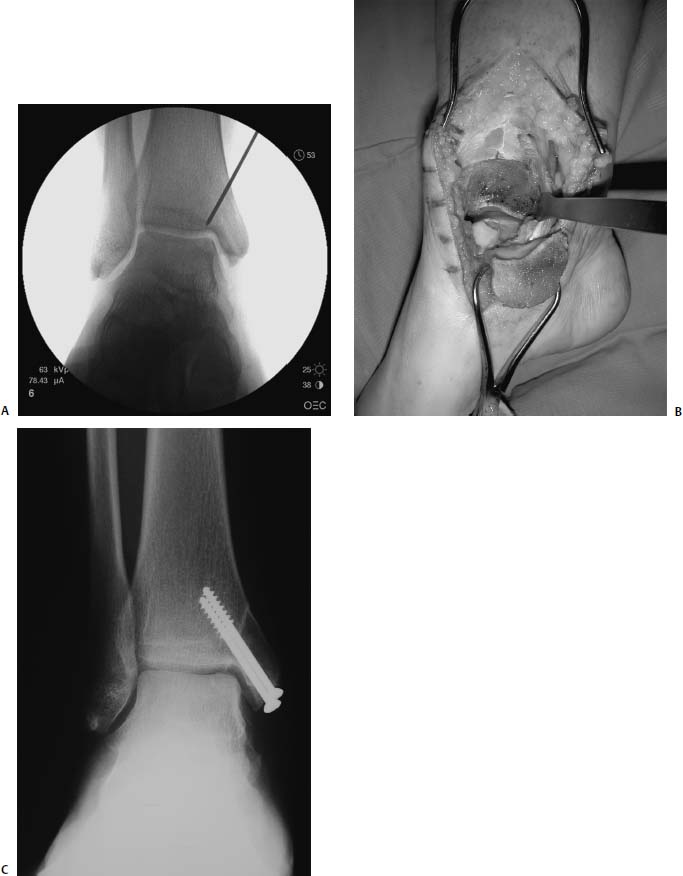
17 Treatment of symptomatic focal talar osteochondral defects has undergone a dramatic evolution over the past decade. Management of symptomatic osteochondral lesions of the talus (OLT) is challenging given the poor healing potential of articular cartilage. The standard of care in surgical management of chronic lesions remains debridement of the OLT in combination with methods of recruiting mesenchymal stems cells to differentiate within the defect (drilling versus microfracture). Intermediate- to long-term results of these techniques have been reported as favorable.1–3 Debridement and drilling or microfracture fails to restore the natural articular cartilage surface, instead filling the void with fibrocartilage. Fibrocartilage is less durable than the adjacent physiologic hyaline cartilage and tends to degenerate with mechanical stress. In smaller diameter lesions (10 mm or less), this is probably well tolerated as mechanical symptoms from impingement of the small lesion are relieved by debridement, and sufficient surrounding hyaline cartilage exists to provide mechanical support. In contrast, larger osteochondral defects (greater than 10 mm) filled with fibrocartilage may provide inadequate mechanical support and may distort the natural viscoelastic properties of the talar dome. Small, nondisplaced acute osteochondral injuries to the talar dome may be observed and are typically excised if displaced. Larger acute osteochondral injuries are probably best treated with internal fixation, particularly if displaced. Frequently, acute injuries are not initially detected, and are thus managed as chronic lesions. Numerous cartilage repair techniques have been described for managing OLTs.4–7 In general, these techniques are reserved for salvage of failed debridement and drilling or microfracture. As for the knee, two fundamentally different treatment principles have recently been popularized as favored cartilage repair procedures: (1) osteochondral autograft/allograft transplantation,6,7 and (2) autologous chondrocyte implantation.4,5 This chapter addresses current concepts in the management of OLT, reviews the prevalence and pathophysiology of OLTs, discusses the diagnosis and staging systems and the operative and nonoperative treatment, and suggests future directions in the management of these lesions. Osteochondral lesions of the talus are rare. Based on the early literature, the reported prevalence of OLTs ranges from 0.09% of all talar fractures8 to 6.5% of ankle sprains.9 The prevalence is likely higher; the use of bone scintigraphy10 and magnetic resonance imaging (MRI)11 in the evaluation of ankle pain has shown that OLTs are more common than previously indicated. The prevalence of bilateral lesions is ~10%.12,13 Medial lesions are more common than lateral lesions, and central lesions are relatively uncommon.14 Arthroscopic evaluation of the talar dome at the time of open reduction and internal fixation of ankle fractures suggests that the prevalence may far exceed what is reported. In a prospective evaluation of 288 ankle fractures assessed arthroscopically, cartilage lesions were identified in 79%.15 Similarly, ankle arthroscopy in patients with lateral ankle ligament injuries reveals a high prevalence of OLTs.16 Ankle articular cartilage is only 1 to 2 mm thick (in comparison, hip and knee cartilage is 3 to 6 mm).17 Degenerative changes in the ankle are rare in the absence of trauma.18 Factors that may protect the ankle include favorable cartilage tensile fracture stresses, favorable responses to degradative proteases, joint congruency, and motion.19 Although cartilage tensile fracture stress decreases exponentially for the hip over time, it decreases only linearly for the talus, and by middle age, ankle articular cartilage typically can withstand greater tensile loads than hip cartilage.20 There also are biochemical advantages in the ankle. In contrast to knee articular cartilage, ankle cartilage expresses minimal RNA for cartilage-degrading enzyme matrix metalloproteinase-8 MMP-821 and exhibits less proteoglycan degradation in response to the catabolic cytokine interleukin-1β (IL-1β) because of a decreased number of IL-1 receptors.22 The advantages described for the intact ankle joint are forfeited following trauma. With a contact area measuring 350 mm2 in the ankle23–25 versus 1120 mm2 in the knee26 and 1100 mm2 in the hip,27 the thinner, stiffer ankle articular cartilage may be less adaptable to articular surface incongruity and increased contact stresses.19 Localized subchondral plate sclerosis (after trauma) increases contact stresses in the cartilage directly overlying the stiffened bone, and associated joint instability may lead to greater damage. Osteochondral lesions of the talus may occur in any location on the talar dome, but typically are observed posteromedially or anterolaterally (Fig. 17–1). Lateral OLTs usually are associated with trauma, whereas medial lesions may be traumatic or nontraumatic.28 Berndt and Harty12 reported that 88% of patients with OLTs had a history of trauma. Moreover, in a cadaver model, they created lateral OLTs with ankle inversion and dorsiflexion and produced medial OLTs with ankle inversion, plantarflexion, and external rotation. Whereas lateral lesions tend to be shallow and wafer-shaped, medial lesions are generally relatively deep and cup-shaped. Canale and Belding29 reported that all lateral lesions and 64% of medial lesions were related to trauma. Likewise, Alexander and Lichtman30 noted that 100% of lateral lesions and 82% of medial lesions were associated with trauma. In a literature review, Flick and Gould31 observed a reported history of trauma associated with 98% of lateral OLT and 70% of medial OLT. Other studies that include arthroscopic evaluation document a history of antecedent trauma in greater than 75%,28,32–34 regardless of the location of the OLT. Similarly, an MRI study suggested 50% of lateral ligament injuries have concomitant OLTs.11 In the absence of trauma, ossification defects, abnormal vasculature, emboli, or endocrine disorders may be responsible for pathologic subchondral fracture of focal areas of the talar dome. Overuse and concentrated pressure increases could produce focal necrosis with formation of a sequestrum or cyst. In the normal ankle joint, considerable stresses are imposed on the medial talar dome; this may explain atraumatic medial dome and bilateral lesions.14,35 FIGURE 17–1 Schematic of an axial view of the talar dome. Osteochondral defects of the talus occur most typically posteromedially or anterolaterally. The methods of diagnosing OLTs have not changed significantly over the past several years. The patient typically reports an ankle injury such as a sprain that fails to heal in the anticipated time course. Persistent pain with activity and accompanying mechanical symptoms, such as popping, catching, and occasional locking are noted. Acute injuries may demonstrate mechanical symptoms consistent with a loose body. The suspected OLT generally can be detected on plain radiographs, but occasionally MRI is necessary to reveal the lesion.36–38 A recent prospective investigation demonstrated that MRI, helical computed tomography (CT), and diagnostic arthroscopy are superior to physical examination and plain radiography in detecting OLTs.39 However, the authors concluded that diagnostic arthroscopy did not outperform MRI or helical CT for detecting or excluding OLTs. Recently, more attention has been given to OLTs associated with ankle fractures and ligamentous ankle injuries, and some surgeons are reporting the benefits of ankle arthroscopy at the time of open reduction and internal fixation of ankle fractures and ligament reconstruction, respectively.15,40,41 Possibly, there is an advantage to the recognition of these lesions in patients with ankle fractures or sprains; fixation or debridement of OLTs may lead to better outcomes following ankle fractures/sprains with associated OLTs. Staging of OLTs is generally based on the original radiographic classification by Berndt and Hardy12: I, small subchondral compression; II, partial fragment detachment; III, complete fragment detachment without displacement; IIIA, complete fragment detachment and rotation without displacement; IV, complete fragment detachment with displacement (Fig. 17–2). An MRI classification has been introduced by Hepple et al37: 1, chondral injury only; 2a, cartilage injury with underlying fracture and surrounding bone edema; 2b, the same as stage 2a but without bone edema; 3, detached but undisplaced fragment; 4, detached and displaced fragment; 5, subchondral cyst formation. Ferkel and Sgaglione42 introduced a CT classification (Fig. 17–3): I, cystic lesion in talar dome with intact roof; IIA, cystic lesion with communication to talar dome surface; IIB, open articular surface lesion with overlying nondisplaced fragment; III, nondisplaced lesion with lucency; IV, displaced fragment. Loomer et al43 added stage V to account for an OLT with a subchondral cyst (Fig. 17–4). FIGURE 17–2 Berndt and Hardy12 staging of osteochondral defects of the talus. (A) Normal. (B) I, small subchondral compression. (C) II, partial fragment detachment. (D) III, complete fragment detachment without displacement. IIIA, complete fragment detachment and rotation without displacement. (F) IV, complete fragment detachment with displacement. FIGURE 17–3 Computed tomography (CT) staging system.43 I, cystic lesion in talar dome with intact roof; IIA, cystic lesion with communication to talar dome surface; IIB, open articular surface lesion with overlying nondisplaced fragment; III, nondisplaced lesion with lucency; IV, displaced fragment. FIGURE 17–4 Osteochondral lesions of the talus (OLT) with subchondral cyst. (A) Plain radiograph suggesting medial OLT. (B) Magnetic resonance imaging (MRI) scan demonstrating cyst with surrounding edema. (C) CT scan defining bony involvement of cyst. Subchondral cysts are difficult to detect by plain radiographs and are frequently difficult to treat. In fact, Kumai et al1 reported that regardless of treatment method, results of management in patients with OLTs associated with subchondral cysts are poor. Robinson et al44 noted a 53% poor outcome in debridement and drilling/curettage of OLTs with subchondral cysts. However, current cartilage resurfacing techniques have shown some promise in management of these deeper lesions.5,7 Because of the difficulty encountered in detecting subchondral cysts by plain radiographs, MRI and/or CT are often considered essential in directing the appropriate treatment of OLTs.36,38,45,46 Despite the advantages of advanced imaging techniques, probably the most accurate staging tool remains arthroscopy, although the limited access to some OLTs during arthroscopy may not provide a full appreciation of an associated subchondral cyst47 (Fig. 17–5). Not all OLTs are symptomatic. Smaller lesions that are relatively stable and produce minimal to no symptoms do not warrant any intervention. Skeletally immature patients retain some healing potential, often prompting immobilization and protective weight-bearing status.48 Most OLTs in skeletally immature patients are caused by trauma. These lesions typically demonstrate favorable healing and outcome. There is a decline with age in the number of pleuripotential mesenchymal cells in the bone marrow, periosteum, and perichondrium. Adult patients rarely demonstrate spontaneous healing. Nevertheless, some OLTs may become asymptomatic despite lack of healing to the surrounding subchondral bone. Surprisingly, a recent report by Shearer et al49 suggests that 71% of stage V lesions (OLTs associated with subchondral cysts) treated without surgery have good-to-excellent results at an average follow-up of 38 months and an average of 88 months after the onset of symptoms. The investigators concluded that (1) the general course of stage V OLT is benign in a majority of patients, (2) nonsurgical treatment of stage V lesions does not lead to the development of advanced osteoarthritis of the ankle, (3) the development of mild radiographic changes of osteoarthritis does not correlate with clinical outcome, (4) most lesions remain radiographically stable, and (5) there is a poor correlation between changes in lesion size and clinical outcome. They acknowledge, however, that patients with larger diameter lesions have a less favorable outcome. FIGURE 17–5 Arthroscopic view of a medial OLT. The cartilage defect is apparent; however, a subchondral cyst cannot be reliably identified by arthroscopy alone. Chondroprotective agents typically utilized to treat knee arthrosis, such as orally administered glucosamine and chondroitin sulfate or injectable hyaluronic acid, may have some role in the management of mildly symptomatic OLT, but to the author’s knowledge, no objective evidence exists to support their administration for OLT.50 A single steroid injection may diminish associated symptoms, but its effect is typically transient. Operative treatment of OLTs is reserved for lesions that fail to respond to nonsurgical measures. However, patients with unstable lesions associated with mechanical symptoms should probably not be subjected to a long course of conservative management, as there is little potential for spontaneous improvement. Debridement and Drilling or Microfracture Arthroscopic debridement and drilling or microfracture remains the standard in operative care of chronic OLT.51 The advantage of debridement and drilling is simplicity. Two to three small arthroscopy portals provide adequate access to the majority of talar dome lesions. Curettage of the unstable cartilage lesions is facilitated by the use of anteromedial, anterolateral, and posterolateral portals. Drilling can be specifically directed to the lesion using a commercially available guide (Microvector, Smith and Nephew, Andover, MA) (Fig. 17–6). Should it be necessary to penetrate the malleoli or distal tibia to access the OLT, the ankle can be dorsiflexed and plantarflexed, so that only one or two drill passes through the intact tibial cartilage is necessary (Fig. 17–7). Small joint awls are becoming available to perform the microfracture technique through the portals, negating the need for transmalleolar drilling.3 Retrograde drilling is also possible to avoid damage to both the intact tibial surface and an intact talar dome with underlying osteochondral lesion.52 A three-dimensional guidance system has been introduced to make drilling these lesions extremely accurate.45,46 In this technique, the OLT is identified by CT or MRI, and under sterile technique a guidewire is placed retrograde into the OLT. The patient is then transferred to the operating room and the guide pin is overdrilled to decompress the OLT. The investigators concluded that the technique is simple, saves time, and avoids damaging an intact cartilage surface overlying the diseased subchondral bone of an OLT. Additional advantages of debridement and drilling/microfracture are (1) it does not compromise the ability to perform a revision surgery should the primary procedure fail, (2) it is typically cost-effective, and (3) there is no concern for donor-site morbidity, unless bone grafting is performed in conjunction with retrograde drilling (however, even this risk can be avoided by using allograft). Most importantly, the results of debridement and drilling are typically favorable. FIGURE 17–6 Drilling can be specifically directed to the lesion using a commercially available guide. Reports reflect the results of previous studies, with good-to-excellent outcomes in 65 to 90% of patients.1,38 In 1986, Parisien33 reported 88% good-to-excellent results in 18 patients with OLT debridement and curettage. Based on a meta-analysis, Tol et al53 suggested that excision and drilling was superior to excision and curettage with good to excellent results of 85% and 78%, respectively. According to a report by Kelberine and Frank,14 better results can be anticipated with management of acute lesions relative to chronic lesions. Schuman et al2 reported good-to-excellent results of debridement and drilling in 86% of primary procedures and 75% in revision procedures at an average follow-up of 4.8 years. Results of retrograde drilling and the microfracture technique have also been reported. Taranow et al52 reported a mean increase in the American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot outcome score of 54 to 83 points in 16 patients treated with retrograde drilling of OLTs at a mean follow-up of 2 years. Thermann and Becher3 managed 32 OLTs (22 acute injuries, 10 chronic lesions) with the microfracture technique; 23 of these patients were examined at a mean follow-up of 2 years. Using a Hannover scoring system for the ankle, good-to-excellent results were noted in 93% of acute lesions and 78% of chronic lesions. The authors also noted that older age (patients >50 years old) was not a limiting factor. Despite encouraging results from multiple investigations, shortcomings of the debridement and drilling technique remain. Physiologic restoration of the defect with hyaline cartilage is not achieved; instead fibrocartilage fills the defect. This is probably acceptable for smaller lesions, but less favorable for larger lesions. Furthermore, the surgical management of the type V lesion43 (subchondral cyst) is less amenable to debridement and drilling as the deeper defect would have only fibrocartilage and lack support of underlying subchondral bone.1,44 Robinson et al44 reported a poor outcome in 53% of OLTs treated with arthroscopic debridement and drilling/curettage. In these cases, bone graft could be added such that fibrocartilage could grow on the surface, but packing bone graft into the defect with the limitations of an arthroscopic approach to the ankle may be prohibitive.54 Nevertheless, bone grafting after excision and curettage has been reported to demonstrate benefit and successful treatment of OLT.55,56 Larger shoulder lesions (transition from superior talar cartilage to medial or lateral cartilage surface) on the talar dome are probably less likely to heal well with debridement and drilling alone. Although fibrocartilage will form, its less favorable mechanical properties may lead to early failure. Finally, Angermann and Jensen57 suggested that results of debridement and drilling deteriorate over time. After noting 85% satisfactory outcome at short-term follow-up of 20 patients managed with debridement and drilling of OLTs, average follow-up of 9 to 15 years revealed that over half had pain and swelling. FIGURE 17–7 The neutral ankle (A) can be dorsiflexed (B) and plantarflexed (C), so that only one or two drill passes through the intact tibial cartilage is necessary. Cartilage Repair Procedures Cartilage repair procedures that involve tissue transplantation include (1) osteochondral autologous transfer system (OATS)(Arthrex, Naples, FL),7,49,58 (2) osteochondral allograft transplantation (fresh or fresh frozen, (3) mosaicplasty,31,59, and (4) autologous chondrocyte implantation (ACI).4,5 Favorable results have been reported in each of the procedures in the knee, and more recently, favorable short-term to intermediate term results are being reported for the talar dome.4,5,7,58–62 The challenge in applying these techniques to the ankle is that access to the talar dome is limited, and often osteotomies are required about the ankle to allow for adequate access for the surgeon to perform the procedure and to allow for proper positioning of instrumentation. Osteochondral Autologous Transfer System/Mosaicplasty/Allograft Osteochondral autologous transfer system (OATS) (Arthrex, Naples, FL) and mosaicplasty (Acufex, Mansfield, MA), which have been used successfully in the knee for many years, have been popularized for talar dome lesions for the past several years. In brief, the technique has three steps: (1) recipient site preparation (removal of an osteochondral plug at the OLT), (2) harvest of a donor-site osteochondral plug from the knee (non-weight-bearing cartilage of the lateral femoral trochlea or notch), and (3) transfer of the donor plug into the recipient site. Unless unique shaping of the osteochondral plug becomes necessary, the donor graft never leaves the harvest tube in the OATS procedure; it is directly implanted into the OLT recipient site after harvest. Osteochondral plug sizes range from 5 to 10 mm, with a 1-mm diameter discrepancy from the recipient site, to create an interference fit of the plug (Fig. 17–8). Results with this technique have been promising in short- to intermediate-term follow-up. Al-Shaikh et al58 reported good-to-excellent results in 88% of previously nonoperated patients and in 91% of patients with failed surgical procedures. The study group comprised 19 patients with an average follow-up of 16 months. Even in the difficult stage V lesions (subchondral cyst), the technique has been shown to be effective. At preliminary follow-up, Scranton and McDermott7 demonstrated an average 27-point increase in the AOFAS score in 10 patients treated for stage V lesions. In the mosaicplasty procedure, the osteochondral graft is routinely removed from the harvesting device and transferred to the device used to implant the graft; osteochondral plug diameters range from 3.5 to 6.5 mm. With smaller diameter grafts, the plugs can be arranged to match the contour of the defect, even if it is located on the shoulder region of the talar dome. Hangody et al59 have reported 94% good-to-excellent results in 36 patients with 2- to 7-year follow-up, applying the mosaicplasty procedure to OLTs greater than 10 mm in diameter. The advantages of these techniques are as follows: (1) it is a single-stage procedure; (2) despite the harvest from the knee, no major donor-site morbidity has been reported; (3) despite osteotomies or ligament releases being required, no major complications have been reported in the surgical exposure of the lesions; and (4) talar dome “shoulder” lesions can be effectively managed with harvest from the edge of the lateral femoral trochlea to match the contour of the cartilage adjacent to the OLT or by harvesting multiple smaller diameter plugs to re-create the physiologic anatomy of the talar shoulder (Fig. 17–9). FIGURE 17–8 Patient with lateral ankle instability and lateral OLT. (A) MRI scan revealing lateral OLT. (B) Lateral approach through the anterior talofibular ligament and osteochondral autograft transplantation system (OATS) instrumentation positioned to insert the osteochondral autograft. (C) Superolateral knee donor site for harvesting osteochondral autograft through arthrotomy. After graft transfer, a modified Brostrom procedure is employed to regain lateral ankle stability. Despite the reported success and low complication rate for the osteochondral autologous transfer procedure in the management of OLTs, some concerns persist: (1) knee and ankle cartilage exhibit different characteristics, (2) donor-site morbidity may occur, and (3) complications may occur from medial and lateral osteotomies. In multiple investigations, Ada Cole and colleagues21,63–69 have demonstrated considerable differences in ankle and knee cartilage, but the significance of this as it pertains to osteochondral transfer between the two joints remains unclear. Although the donor-site morbidity appears low, regardless of whether the osteochondral graft is harvested arthroscopically or through a small arthrotomy, it does exist. For this reason, some investigators have attempted to transfer local grafts about the ankle, and results using this technique are promising.70,71 Sammarco and Makwana71 reported a significant improvement in the AOFAS score at an average follow-up of 25 months in 12 patients treated with this method. Use of fresh or fresh-frozen osteochondral allograft eliminates the risk of donor-site morbidity as well. Brage72 reported promising results using fresh talar dome osteochondral allograft at short- to intermediate-term follow-up. Gross et al73 demonstrated that the majority (six of nine) of these grafts incorporate and function reasonably well at longer-term follow-up. The risk of disease transmission appears to be low, but the use of fresh grafts may require a dedicated team at a well-equipped institution to make such transplantation practical. Furthermore, although donor-site morbidity is nonexistent, osteotomies about the ankle are generally still necessary, no different from the autologous procedures described above. FIGURE 17–9 Medial talar dome shoulder defect managed with the mosaicplasty technique (described by Laslo Hangody, M.D.). (A) Schematic diagram shows how to position autologous osteochondral plugs. (B) Intraoperative photo through the medial malleolar osteotomy. (Courtesy of Laslo Hangody, M.D.) Autologous Chondrocyte Implantation Autologous chondrocyte implantation (ACI) has gained recent popularity in treating chondral and osteochondral lesions in the knee. Given the success in the knee, the technique has been applied to the ankle as well. In brief, the procedure is performed in stages. Stage one consists of (1) harvest of a small amount of nonweight-bearing articular cartilage, typically from the knee; and (2) culturing of these chondrocytes. Stage two consists of (1) debridement of the OLT, (2) harvest of a periosteal flap, (3) attachment of the periosteal flap to the intact articular cartilage adjacent to the OLT, and (4) injection of the cultured chondrocyte suspension under the periosteal flap in the cartilage defect. The procedure’s second stage is rather tedious and typically requires an osteotomy to access the lesion, unless the ankle pathology includes lateral ankle instability. Suturing of the periosteal flap must be performed in a restricted space despite osteotomy. Advantages are as follows: (1) large defects can be easily addressed with this technique, as there really is no limit to the size defect that can be repaired; (2) the periosteal flap is harvested from the adjacent distal tibia; and (3) with careful suture technique, shoulder lesions can also be treated. Disadvantages are as follows: (1) the procedure is Food and Drug Administration (FDA)-approved only for the knee, not for the ankle (as of March 2005); (2) the fee for the chondrocyte culturing process is considerable; and (3) the procedure must be performed in a staged fashion to allow time for the chondrocyte culture. A further concern has been that the technique could not effectively address type V lesions (subchondral cysts) because (1) chondrocytes die if exposed to bleeding; and (2) deeper lesions cannot simply be filled with chondrocytes, but require subchondral support, particularly large diameter lesions. However, advanced techniques of ACI have successfully been applied to stage V lesions as well. A “sandwich technique” has been employed in which (1) bone graft is packed within the defect after the OLT is debrided and the deeper bone is drilled, (2) the first periosteal flap is sutured on the bone graft with the cambium layer facing the joint, (3) a second periosteal flap is sutured in the traditional fashion at the level of the healthy adjacent articular cartilage with the cambium layer away from the joint, and (4) the chondrocyte suspension is injected between the periosteal flaps74 (Fig. 17–10). Results of ACI are favorable in the ankle. At the time of this publication, Lars Peterson, who developed the procedure, had performed over 30 autologous chondrocyte implantations for OLTs. His group reported results on the first 14 patients, with an average follow-up of 45 months: 12 patients are considered improved, and 11 have good-to-excellent outcomes.5,75 Giannini et al4 reported an improvement in the average AOFAS anklehindfoot score from 32 to 91 points in eight patients examined at a mean follow-up of 26 months. Histologic examination of biopsies obtained at second-look arthroscopies performed at 12 months suggested physiologic type 2 hyaline cartilage (cartilage thickness, chondrocyte viability, staining) in all eight specimens.4,59 In Europe, a matrix-induced autologous chondrocyte implantation (MACI) system has been developed (Verigen AG, Leverkusen, Germany). The use of the combination of MACI and a purified collagen membrane to treat OLTs has shown favorable preliminary results and may allow for similar results to ACI with less extensive surgical exposure. FIGURE 17–10 Autologous chondrocyte implantation for medial talar dome defect performed through a medial malleolar osteotomy. (A) Injection of cartilage cells (inset close-up). (B) Final view after periosteal flap sealed with fibrin glue. (C) Image of “sandwich technique.” Two periosteal flaps are harvested from distal medial tibia and a posteromedial defect is visualized through the medial malleolar osteotomy. Note the drill hole in the distal tibia to harvest bone graft to fill the subchondral cyst. Osteotomies A major concern remains the extensive surgery that the ankle must endure to allow the surgeon adequate exposure to place these osteochondral grafts. Not all lesions require osteotomies; some anterolateral, anteromedial, and posteromedial lesions can be addressed with ankle hyperplantarflexion or hyperdorsiflexion and capsular releases and/or creation of troughs in the distal tibia. Typically, however, medial or lateral malleolar osteotomies are required to permit satisfactory exposure. Risk of nonunion or malunion has prompted development of alternative osteotomies/approaches that may be less morbid. Oznur76 has described a medial malleolar window; Tochigi et al77 introduced an anterolateral distal tibial osteotomy, and Sammarco and Makwana71 have utilized a replaceable anterior tibial plafond bone block. Each of these techniques shows promise, with the medial malleolar window potentially allowing safer access to medial talar dome lesions, the anterolateral distal tibial osteotomy improving access to centrolateral OLTs, and the anterior tibial plafond bone block permitting improved exposure to multiple areas of the talar dome. A fibular window and door have also been described by Hansen78 and Allen and DiGiovanni.79 A posteromedial arthrotomy through the posterior tibialis tendon sheath has also been described by Bassett et al.80 A maximum dorsiflexion lateral radiograph is required in the preoperative workup to ensure the postero- or centromedial lesion can be accessed via this approach. The size of the lesion and the ability to place required instrumentation through this arthrotomy need to be considered. This technique does avoid the possibility of complications from a medial malleolar osteotomy. Multiple variations of the medial malleolar osteotomy have been described, including chevron, step-cut, and oblique variants. This osteotomy enables access to the central and posterior lesions on the medial talus (Fig. 17–11). Technique pearls include the following: (1) Perpendicular instrument access to the lesion is not possible unless the line of the osteotomy is adequately vertical. It must then enter the joint at the junction of the medial malleolus and tibial plafond, so as to not disrupt the weight-bearing surface of the plafond. To avoid damage to the articular cartilage, the osteotomy should be completed with an osteotome. (2) The more oblique the cut, the more eversion and abduction of the talus are needed. The lateral capsule and lateral malleolus are the limiting factors to talar excursion and visualization. (3) The more vertical the cut, the more vertical shear is placed on the fixation during active range of motion through the posterior tibial and Achilles tendons. (4) Fixation of the malleolar fragment should begin with predrilling and tapping of the screw holes prior to osteotomy to avoid loss of orientation and to ensure accurate reduction upon application of final fixation. (5) Necessary and thus available fixation should include 4.0-mm malleolar screws, 2.7- and 3.5-mm cortical screws, and a low-contour plate if apical compression is required.81 Even though the cartilage repair procedures described above are promising in OLT management, no long-term clinical studies are available to demonstrate the efficacy of these techniques. Arthroscopic evaluation and biopsy of the cartilage repair tissue is most definitive in defining the success of the procedure,59 but MRI shows promise in follow-up evaluation.36,38,54 The shoulder lesion in particular remains a difficult problem. Hangody et al59 and Peterson et al60 demonstrated techniques for this difficult lesion, but it is hoped that refinement of old and newer techniques will provide better outcomes. As for the knee, ligament stability and joint alignment are essential to avoid excessive contact and shear stresses across the repaired cartilage surfaces. Although distal femoral and high tibial osteotomies are being performed with greater frequency to unload knee compartments with repaired osteochondral defects, the need for periarticular osteotomies about the ankle to unload repaired OLTs has not yet been defined. Clearly there is a role for stabilization of ligamentous instability around the ankle joint to protect the cartilage healing potential just as in the knee. Pleuripotent cells from other sources may have better potential than mature chondrocytes to repair osteochondral defects and may differentiate and organize into appropriate zones to re-create physiologic subchondral and chondral anatomy. Combined with structural matrices (to support zonal organization of articular cartilage) and growth factors (to promote cell differentiation and bonding of transplanted tissue to adjacent normal cartilage), pleuripotent cells may be the future to promoting a more natural healing response for OLTs. Mesenchymal progenitor cells may allow for repair of subchondral and cartilage defects. These cells may be cultured similarly to chondrocytes as in autologous chondrocyte transfer. Current research has focused on development of matrices that could serve as effective delivery systems for these progenitor cells. To our knowledge, this technique has yet to be described specifically for OLT. Fibrin clot containing mitogenic growth factors (basic fibroblast growth factor, transforming growth factor-(1, epidermal growth factor, insulin-like growth factor-1, and growth hormone) has been used as a scaffold for the migration of reparative cells.82 FIGURE 17–11 Medial malleolar osteotomy. (A) Intraoperative fluoroscopy to determine the proper plane for the oblique osteotomy. (B) Intraoperative photo of exposure of a large OLT obtained with a medial malleolar osteotomy. (C) Postoperative radiograph with healed medial malleolar osteotomy. Artificial structural matrices are designed to act as scaffolding for cartilage growth within an osteochondral defect. The ideal matrix has yet to be developed, but it should meet the following requirements: (1) it should have appropriate porosity to allow for cell migration; (2) it should serve as a carrier for hormonal substances that direct cell maturation and proliferation; (3) it should allow for cell adhesion; (4) it should provide a malleable contour permitting maximum contact with the host tissue; (5) it should enhance the binding of the repair tissue to the host tissue; (6) it should have sufficient biodegradability to allow for remodeling of the repair tissues; (7) it should provide matrix cohesiveness; (8) it should have sufficient resiliency to allow for dynamic and static deformation; and (9) it should bond adequately to prevent displacement. Absorbable polymers that can be locally implanted into defects would likely be most useful. A collagen sponge has been developed that can serve as a scaffold in an osteochondral defect. A polyglycolic acid implant has been utilized in animal studies. Carbon fiber scaffolds have also been evaluated, though they seem to provide only fibrous tissue within osteochondral defects. Teflon, Dacron, Gore-Tex, and several other polymers have been studied, although none has demonstrated the ability to generate healing of cartilage in the animal models employed. Until gene-modified tissue engineering becomes available to activate an appropriate healing response in humans, osteochondral transfer and autologous chondrocyte implantation represent attractive alternatives. REFERENCES 1. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am 1999;81:1229–1235 2. Schuman L, Struijs PA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus: results at follow-up at 2 to 11 years. J Bone Joint Surg Br 2002;84:364–368 3. Thermann H, Becher C. [Microfracture technique for treatment of osteochondral and degenerative lesions of the talus: 2-year results of a prospective study.] Unfallchirurg 2004;107:27–32 4. Giannini S, Buda R, Grigolo B, et al. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int 2001;22:513–517 5. Peterson L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 2003;8:291–303 6. Hangody L, Kish G, Modis L, et al: Mosaicplasty for the treatment of osteochondritis dissecans of the talus; two to seven year results in 36 patients. Foot Ankle Int 2001;22:552–558 7. Scranton PE Jr, McDermott JE. Treatment of type V osteochondral lesions of the talus with ipsilateral knee osteochondral autografts. Foot Ankle Int 2001;22:380–384 8. Coltart WD. Aviator’s astragalus. J Bone Joint Surg Am 1952;34:545–566 9. Bosien WR, Staples OS, Russell SW. Residual disability following acute ankle sprains J Bone Joint Surg Am 1955;37:1237–1243 10. Urman M, Ammann W, Sisler J, et al. The role of bone scintigraphy in the evaluation of talar dome fractures. J Nucl Med 1991;32:2241–2244 11. Hepple S, Winson IG, Glew D. Lateral ligament injuries and osteochondral lesions in MRI of the ankle. In: Programs and Abstracts of the 13th annual summer meeting of the American Orthopaedic Foot and Ankle Society, Monterey, CA, 1997 12. Berndt A, Hardy M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am 1959;41:988–1020 13. Blom JM, Strijk SP. Lesions of the trochlea tali: osteochondral fractures and osteochondritis dissecans of the trochlea tali. Radiol Clin (Basel) 1989;44:387–399 14. Kelberine F, Frank A. Arthroscopic treatment of osteochondral lesions of the talar dome: a retrospective study of 48 cases. Arthroscopy 1999;15:77–91 15. Hintermann B, Regazzoni P, Lampert C, et al: Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br 2000.;82:345–351 16. Takao M, Ochi M, Uchio Y, Naito K, Kono T, Oae K. Osteochondral lesions of the talar dome associated with traum. Arthroscopy 2003;19:1061–1067 17. Athanasiou KA, Niederauer GG, Schenck RC Jr. Biomechanical topography of human ankle articular cartilage. Ann Biomed Eng 1995;23:697–701 18. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in knee and ankle joints. Semin Arthritis Rheum 1997;26:667–674 19. Buckwalter JA, Saltzman CL. Ankle osteoarthritis: distinctive characteristics. Instr Course Lect 1999;48:233–241 (AAOS) 20. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta 1991;1075:223–227 21. Chubinskaya S, Huch K, Mikecz K, et al. Chondrocyte matrix metalloproteinase-8: up-regulation of neutrophil collagenase by interleukin-1 beta in human cartilage from knee and ankle joints. Lab Invest 1996;74:232–240 22. Hauselmann HJ, Fletcenmacher J, Gitelis SH, et al. Chondrocytes from human knee and ankle joints show differences in response to IL-1 and IL-1 reception inhibitor. Trans Orthop Res Soc 1993;17:710 23. Alexander AH, Lichtman DM. Surgical treatment of transchondral talar dome fractures: long-term follow-up. J Bone Joint Surg Am 1980;62:646–652 24. Beaudoin AJ, Fiore SM, Krause WR, et al. Effect of isolated talocalcaneal fusion on contact in the ankle and talonavicular joints. Foot Ankle 1991;12:19–25 25. Kimizuka M, Kurosawa H, Fukubayashi T. Loadbearing pattern of the ankle joint: contact area and pressure distribution. Arch Orthop Trauma Surg 1980;96:45–49 26. Ihn JC, Kim SJ, Park IH. In vitro study of contact area and pressure distribution in the human knee after partial and total meniscectomy. Int Orthop 1993;17:214–218 27. Brown TD, Shaw DT. In vitro contact stress distributions in the natural human hip. J Biomech 1983;16:373–384 28. Van Buecken K, Barrack RL, Alexander AH, et al. Arthroscopic treatment of transchondral fractures of the talar dome. Am J Sports Med 1989;17:350–355 29. Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am 1980;62:97–102 30. Alexander AH, Lichtman DM. Surgical treatment of transchondral talar dome fractures: long-term follow-up. J Bone Joint Surg Am 1980;62:646–652 31. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle Int 1985;5:165–185 32. Baker CL, Andrews JR, Ryan JB. Arthroscopic treatment of transchondral talar dome fractures. Arthroscopy 1986;2:82–87 33. Parisien JS. Arthroscopic treatment of osteochondral lesions of the talus. Am J Sports Med 1986;14:211–217 34. Pritsch M, Hroashovski H, Farine I, et al. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am 1986;68:862–865 35. Vrahas M, Fu F, Veenis B. Intraarticular contact stress with simulated ankle malunions. J Orthop Trauma 1999;82:159–166 36. Assenmacher JA, Kelikian AS, Gottlob C, Kodros S. Arthroscopically assisted autologous osteochondral transplantation for osteochondral lesions of the talar dome: an MRI and clinical follow-up study. Foot Ankle Int 2001;22:544–551 37. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int 1999;20:789–793 38. Higashiyama I, Kumai T, Takakura Y, Tamail S. Follow-up study of MRI for osteochondral lesion of the talus. Foot Ankle Int 2000;21:127–133 39. Verhagen RA, Maas M, Dijkgraaf MG, et al. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br 2005;87:41–46 40. Loren GJ, Ferkel RD. Arthroscopic assessment of occult intra-articular injury in acute ankle fractures. Arthroscopy 2002; 18:412–421 41. Hintermann B, Bos A, Schafer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med 2002;30:402–409 42. Ferkel RD, Sgaglione N. Arthroscopic treatment of osteochondral lesions of the talus: long-term results. Trans Orthop Res Soc 1990;14:172–178 43. Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med 1993;21:13–19 44. Robinson DE, Winson IG, Harries WJ: Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg 2003;85-B:989–993 45. Bale RJ, Hoser C, Rosenberger R, Rieger M, Benedetto KP, Fink C. Osteochondral lesions of the talus: computer-assisted retrograde drilling—feasibility and accuracy in initial experiences. Radiology 2001;218:278–282 46. Fink C, Rosenberger RE, Bale RJ, et al. [Computer-assisted retrograde drilling of osteochondral lesions of the talus.] Orthopade 2001;30:59–65 47. Schimmer RC, Dick W, Hintermann B. The role of ankle arthroscopy in the treatment strategies of osteochondritis dissecans lesions of the talus. Foot Ankle Int 2001;22:895–900 48. Higuera J, Laguna R, Peral M, Aranda E, Soleto J. Osteochondritis dissecans of the talus during childhood and adolescence. J Pediatr Orthop 1998;18:328–332 49. Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int 2002;23:651–654 50. Marshall KW. Intra-articular byaluronan therapy. 2003;8:221 51. Struijs PA, Tol JL, Bossuyt PM, Schuman L, van Dijk CN. [Treatment strategies in osteochondral lesions of the talus. Review of the literature.] Orthopade 2001;30:28–36 52. Taranow WS, Bisignani GA, Towers JD, et al. Retrograde drilling of osteochondral lesions of the medial talar dome. Foot Ankle Int 1999;20:474–480 53. Tol JL, Struijs PA, Bossuyt PM. Treatment strategies in osteochondral defects of the talar dome: A systematic review. Foot Ankle Int 2000;21:119–126 54. Lahm A, Erggelet C, Steinwachs M, Reichelt A. Arthroscopic management of osteochondral lesions of the talus: results of drilling and usefulness of magnetic resonance imaging before and after treatment. Arthroscopy 2000;16:299–304 55. Bruns J. Osteochondrosis dissecans. Orthopade 1997;26:573–584 56. Draper SD, Fallat LM. Autogenous bone grafting for the treatment of talar dome lesions. J Foot Ankle Surg 2000;39:15–23 57. Angermann P, Jensen P. Osteochondritis dissecans of the talus: long-term results of surgical treatment. Foot Ankle 1989; 10:161–163 58. Al-Shaikh RA, Chou LB, Mann JA, Dreeben SM, Prieskorn D. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int 2002;23:381–389 59. Hangody L, Kish G, Modis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int 2001;22:552–558 60. Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 2003;8:291–303 61. Ferkel RD. AAOS Annual Meeting, 2004(publication pending) 62. Hangody L. The mosaicplasty technique for osteochondral lesions of the talus. Foot Ankle Clin 2003;8:259–273 63. Chubinskaya S, Kuettner KE, Cole AA. Expression of matrix metalloproteinases in normal and damaged articular cartilage from human knee and ankle joints. Lab Invest 1999;79:1669–1677 64. Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res 2002;20:526–534 65. Homandberg GA, Kang Y, Zhang J, Cole AA, Williams JM. A single injection of fibronectin fragments into rabbit knee joints enhances catabolism in the articular cartilage followed by reparative responses but also induces systemic effects in the non-injected knee joints. Osteoarthritis Cartilage 2001;9:673–683 66. Kang Y, Koepp H, Cole AA, Kuettner KE, Homandberg GA. Cultured human ankle and knee cartilage differ in susceptibility to damage mediated by fibronectin fragments. J Orthop Res 1998;16:551–556 67. Koepp H, Eger W, Muehleman C, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci 1999;4:407–412 68. Schumacher BL, Su JL, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec 2002;266:241–248 69. Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res 2000;18:739–748 70. Lee MS. Anterior talar dome as an alternative donor site for osteochondral transplantation for medial talar dome lesions. Clin Podiatr Med Surg 2001;18:545–549 71. Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int 2002;23:693–698 72. Brage M. Osteochondral grafting of osteochondral lesions of the talus. In: Proceedings of the International Federation of Foot and Ankle Societies: Triennial Scientific Meeting, 2002;146–147 73. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int 2001;22:385–391 74. Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med 1999;18:13–44 v–vi. 75. Peterson L. Treatment of Cartilage Injuries in the Ankle. In: Proceedings of the International Federation of Foot and Ankle Societies: Triennial Scientific Meeting, 2002;147–148 76. Oznur A. Medial malleolar window approach for osteochondral lesions of the talus. Foot Ankle Int 2001;22:841–842 77. Tochigi Y, Amendola A, Muir D, Saltzman C. Surgical approach for centrolateral talar osteochondral lesions with an anterolateral osteotomy. Foot Ankle Int 2002;23:1038–1039 78. Hansen ST Jr. Functional Reconstruction of the Foot and Ankle. Philadelphia: Lippincott Williams & Wilkins, 2000:494–497 79. Allen SD, DiGiovanni CW. Distal fibular window osteotomy for exposure of lateral talar osteochondral lesions. Tech Foot Ankle Surg 2003;2:129–134 80. Bassett FH, Billys JB, Gates HS. A simple surgical approach to the posteromedial ankle. Am J Sports Med 1993;21:144–147 81. Kish G. Mosaicplasty for osteochondral lesions of the talus. In: Masters Techniques in Orthopaedic Surgery: The Foot and Ankle, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2002:643–664 82. Hunziker EB, Kapfinger E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br 1998;80:144–149
Osteochondral Lesions of the Talus
 Prevalence and Pathophysiology
Prevalence and Pathophysiology
 Ankle Joint Characteristics
Ankle Joint Characteristics
 Etiology
Etiology
 Diagnosis and Staging
Diagnosis and Staging
 Nonoperative Treatment
Nonoperative Treatment
 Surgical Treatment
Surgical Treatment
 Future Directions
Future Directions
< div class='tao-gold-member'>
Osteochondral Lesions of the Talus
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


