19
Osteochondral Defects in the Elbow
Treatment of articular cartilage injuries remains difficult and frustrating in virtually every joint in which these maladies occur. This is particularly true of young athletes who continue to place high demands on their joints. Due to the poor biologic properties of articular cartilage, these lesions have minimal potential for healing, making them susceptible to progression with repetitive trauma. Many of the injuries develop prior to skeletal maturity and are underappreciated or missed. Osteochondritis dissecans is the prototypical chondral lesion of the elbow affecting young athletes. This condition is seen primarily in throwing athletes as well as gymnasts, although it may occur in any number of sports.
When treating osteochondral injuries, consideration must be given to the skeletal maturity of the individual. Treatment differs to some extent between skeletally immature and mature patients. This chapter focuses on the etiology, presentation, imaging, and treatment of osteochondritis dissecans, as this is perhaps the most difficult articular problem to treat in the skeletally immature athlete’s elbow.
Treatment has evolved from removal of loose bodies, first described more than 150 years ago,1 to nonoperative treatment,2–4 arthroscopic debridement,5,6 arthroscopic subchondral drilling,3,4,7 open drilling,2,8 abrasion chondroplasty,9 and internal fixation with bone pegs,7 bioabsorbable or metal screws,10,11 pull-out wire,12 compression staples,13 and most recently tissue engineered cartilage gels.14 Most authors would agree there is no indication for reduction and fixation of loose bodies. Excision of the fragment only with debridement of the bed is the mainstay of treatment.2–8 Long-term results have shown that about half of the affected adolescents will develop symptomatic degenerative joint disease.4 Magnetic resonance imaging (MRI) with and without contrast has been shown to help with early detection, which may allow for earlier intervention and improved long-term outcome.15 Although most of the attention in the orthopaedic literature focuses on osteochondritis dissecans of the capitellum, the process has been reported in the trochlea,16 radial head, and olecranon.17,18
Reconstructive options such as interposition arthroplasty and total elbow arthroplasty are not discussed here, as these are primarily salvage procedures not designed for the athletic population, but they remain an important part of the armamentarium of elbow surgeons.
 Etiology
Etiology
Osteochondritis dissecans (OCD) occurs predominantly in immature athletes and is rarely found in adults, although the sequelae are often seen in adults. It is a localized injury or condition to the subchondral bone resulting in loss of support for the overlying articular cartilage, which leads to breakdown and fragmentation of the cartilage and underlying bone.19 Various theories regarding etiology have been proposed, but no single etiology is universally accepted.20 König21 is credited with the original description and naming of the lesion. The term is somewhat inaccurate as the name implies inflammation of the bone and cartilage. No inflammatory cells have been shown on histologic sections of excised fragments or surrounding synovium.22,23 However, dissecans comes from the Latin dissec-, meaning to separate, and it accurately describes the separation of osteochondral fragments seen in the late stages of the process.
There are two similar disorders of the humeral capitellum that occur in immature individuals with similar radiographic findings: Panner’s disease and OCD. The age at presentation and the prognosis are different, and therefore they should be distinguished as separate but related entities. Panner’s disease typically presents in patients between 7 and 12 years of age, with a peak age of 9 years.24,25 It is not associated with repetitive trauma, and it demonstrates flattening and patchy sclerosis of the entire humeral capitellum on x-ray. The capitellum reconstitutes with time and results in no demonstrable long-term sequelae.26,27 OCD, in contradistinction, presents between 11 and 15 years of age and is associated with repetitive trauma, especially baseball and gymnastics, but has been seen in ice and roller hockey. Radiographically, the lesion is more focal with capitellar rarefaction (Fig. 19–1). As the lesion progresses, capitellar flattening and subsequent fragmentation occur. Long-term degenerative joint disease affects nearly half of the elbows.4,27–29
Most authors agree that a combination of injury from repetitive trauma and a tenuous blood supply to the humeral capitellum leads to OCD.20 Schenk et al30 have demonstrated biomechanical differences between the capitellar and the radial head articular cartilage, which may play a role in the genesis of OCD. Significantly stiffer radial head cartilage compared with the lateral capitellum creates a mechanical mismatch, which may cause injury to the capitellum that explains the creation of an OCD lesion.20,30 Some individuals may have a genetic predilection to OCD, as evidenced by reports of bilateral and multiple joint involvement.10,18 Review of the literature reveals that OCD of the capitellum predominantly affects the dominant arm of Little League male pitchers and helps support an etiology with trauma as a major component2–4,6,15,22,31–33 High stresses are applied to the elbow during early and late cocking of the throwing cycle. A significant distraction force is applied to the medial aspect of the elbow.32,34 Compression and shear forces occur at the radiocapitellar articulation in late cocking.33–37 Reports of OCD in young female gymnasts seems to corroborate this assertion.2 In gymnastics, the elbow becomes a weight-bearing joint as the radiocapitellar joint transmits 60% of the force across the elbow.2,38
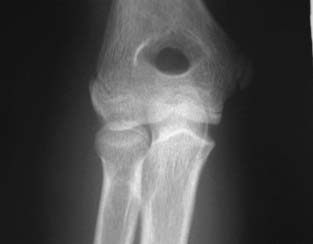
FIGURE 19–1 A 14-year-old female roller hockey player demonstrating capitellar rarefaction of early stable osteochondritis dissecans (OCD) on 45-degree flexion anterior posterior view.
The ischemic component is based on Haraldsson’s39 description of the vascular anatomy supplying the distal humerus and in particular the capitellum. The capitellar epiphysis receives blood from only one or two isolated vessels that enter posteriorly and traverse the cartilaginous capitellum. No metaphyseal collateral flow exists, resulting in a tenuous blood supply. Thus, the ability of the epiphysis to heal between traumatic events may be limited, rendering it susceptible to osteonecrosis. The histopathology is more consistent with necrosis than an inflammatory event.23 Hyperemia and edema are the earliest changes. Loss of subchondral bone support results in articular cartilage breakdown and the formation of loose bodies.26 Takahara et al3,15 believe that the new healing subchondral bone exposed to stress will fracture, leading to articular cartilage fragmentation. Furthermore, removal of stress early in the process can prevent progression of the disease.
 History
History
The typical patient is an adolescent baseball pitcher between 11 and 15 years of age who has been pitching for 3 to 5 years prior to the onset of symptoms.15 Patients often seek medical attention only after several months of pain.2,15 Pain is often localized to the lateral aspect of the elbow and relieved by rest. Catching or locking of the elbow are late symptoms and are indicative of articular cartilage fragmentation and loose body formation.4,15 It is important to note that pain may not be present or may be poorly localized, as the presenting symptoms can be variable and benign in appearance.
 Examination
Examination
Tenderness laterally over the radiocapitellar joint is often present but may be poorly localized. Loss of extension is more common than loss of flexion; however, early in the disease process no motion loss may be present.15 Provocative maneuvers include the active radiocapitellar compression test,19 which involves having the patient pronate and supinate the forearm in full extension. Compression across the radiocapitellar joint from muscular forces may reproduce symptoms.19
 Diagnostic Evaluation
Diagnostic Evaluation
Plain radiographs are the initial diagnostic test of choice. Very early in the disease process, radiographs may be negative or show very subtle changes.15 Anterior-posterior radiographs with the elbow in full extension may not demonstrate the lesion. Takahara et al3 have shown that obtaining anterior-posterior radiographs in 45 degrees of flexion is more helpful (Fig. 19–1). As the disease progresses, flattening of the capitellar subchondral bone along with subchondral bone rarefaction and isolation of the OCD fragment by the zone of rarefaction are seen and loose body formation occurs.3,40 The classic lesion occurs on the anterolateral aspect of the capitellum. Widening of the radial head and medial osteophyte formation are seen late in the disease process. The traditional classification on which discussion and treatment have been based is an adaptation of Minami et al’s description.7,41 Lesions are graded on the anterior-posterior view of the elbow and stratified into three grades. Grade I lesions demonstrate a translucent cystic shadow in the lateral or middle capitellum. Grade II lesions demonstrate a clear zone, or a split line is seen between the lesion and the adjacent subchondral bone. Grade III elbows have loose bodies.
Three basic types of lesions have been identified: stable, unstable but attached, unstable and loose (i.e., loose bodies). These types correspond roughly to the radiographic classification. Stable lesions by definition are in situ and have intact articular cartilage. Unstable lesions are those in which the overlying articular cartilage is broken. The lesions may remain attached as in situ unstable lesions or they may detach and become loose bodies.26,42
The size of the lesion according to Takahara et al4 has predictive value and should be evaluated. The defect is sized on the anterior-posterior radiograph as a percent of the entire capitellum (Fig. 19–2A). Additionally, the defect angle can be measured on the lateral x-ray (Fig. 19–2B). A large defect measured >70% with a defect angle of 90 degrees. A small defect measured <55% with a defect angle of <60 degrees. All others were classified as moderate. According to Takahara et al, all small lesions do well and all large lesions do poorly. Moderate-size lesions have variable outcome.
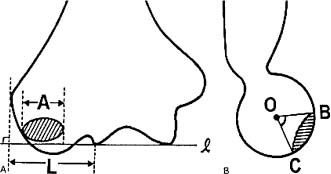
FIGURE 19–2 Diagrams of (A) anteroposterior (AP) and (B) lateral radiographs showing osteochondral defect in the capitellum. (A) Defect size (%) = A/L × 100. A = length (mm) of the osteochondral defect, paralleling the line l; L = length of capitellum (mm). (B) Defect angle B = superior aspect, C = inferior aspect of the defect, and O = the center of the capitellum. (From Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop 1999;363: 108–115, with permission.)
In an attempt to elucidate the early pathologic changes in the humeral capitellum, Takahara et al15 used MRI and ultrasound to evaluate the early changes in OCD, prior to fragmentation. They had minimal radiographic changes best seen on 45 degrees flexion view. Demonstrable changes were seen only on T1-weighted MRI scans and consisted of a low signal intensity at the capitellar surface. In contrast to more advanced lesions no abnormality was seen on T2-weighted images. Ultrasound was used to confirm that capitellar flattening was present. Having the patient refrain from throwing (i.e., the offending force) was necessary to obtain healing. Takahara et al’s series was limited to three patients and therefore should be considered presumptive evidence. Early stable lesions can be seen as an increased signal on two-dimensional (2D) fast spin-echo sequences (Fig. 19–3).
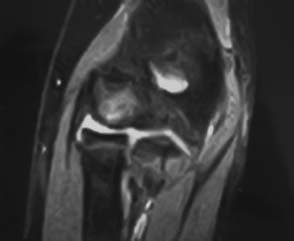
FIGURE 19–3 Magnetic resonance imaging (MRI) (corresponding to x-ray in Fig. 19–1) without contrast of an early stable OCD lesion in an immature elbow.
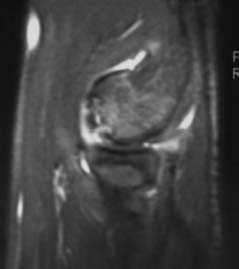
FIGURE 19–4 MRI with intraarticular gadolinium of an unstable OCD lesion in an elbow, sagittal view. Notice dye tracking into subchondral cleft indicating an unstable lesion. (Photo courtesy of Karenze Chan, M.D.)
The normal MRI anatomy of the elbow has been reported as well as the MRI findings in typical OCD of the elbow.43–47 Loose in situ lesions may be diagnosed by the appearance of a cyst under the lesion.45 Staging accuracy can be improved with MR arthrography.48 Intraarticular contrast has been used in an attempt to assess stability of the OCD fragment as it relates to the integrity of the articular surface and loose body formation. Both saline and dilute gadolinium have been used.47 Dye tracking into the interface between the fragment and proximal bone suggests a break in the articular surface and hence an unstable lesion44 (Figs. 19–4 and 19–5). However, not all loose fragments demonstrate these MRI findings and may be mistaken for a stable fragment.40
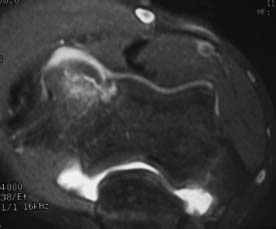
FIGURE 19–5 MRI with intraarticular gadolinium of an unstable OCD lesion in an elbow. Notice the dye tracking between the OCD fragment and the underlying subchondral bone and into the subchondral cleft. Image corresponds to sagittal MRI in Fig. 19–4. (Photo courtesy of Karenze Chan, M.D.)
The use of intravenous gadopentetate-dimeglumine has been described in an attempt to evaluate the stability and viability of attached OCD fragments and may improve the diagnostic and prognostic capabilities of MRI.40 Fragment enhancement following intravenous contrast (i.e., gadopentetate-dimeglumine) signifies blood supply and hence viability of the fragment. A diffusely enhancing lesion at the fragment subchondral bone interface suggests granulation tissue is present, indicating that the fragment is unstable. This technique, however, has limited potential to assess the integrity of the articular surface in comparison to intraarticular contrast.
A potential MRI pitfall is the pseudodefect of the capitellum that is occasionally mistaken for OCD.45 The normal articular portion of the capitellum is an anteriorly directed hemisphere. The pseudodefect occurs at the posteroinferior junction of the articular and nonarticular portions for the capitellum.45,49 True OCD of the capitellum is directed anteriorly.
Computed tomography (CT) arthrography has also been employed with intraarticular gadolinium to evaluate articular cartilage.28 It is perhaps better than MRI at detecting loose bodies, although relatively less effective at assessing the articular cartilage.
 Review of the Literature
Review of the Literature
Interpreting the literature is difficult. Studies often do not distinguish between very early, early, and late presenting OCD. No universally accepted classification exists, and not all studies include MRI findings. In addition, surgical techniques have changed dramatically over the past 15 years. Therefore, comparisons of more recent studies to historical studies are difficult.
Central to the treatment of OCD fragments is their size, stability, viability, and location. To date only one study has addressed the viability of an OCD fragment40; these results, however, are preliminary. None of the other larger studies in the literature incorporate this issue into their treatment algorithm. Presumably, a viable fragment would have the best chance of healing.
In 1985, McManama et al8 reported on 14 adolescents with OCD of the elbow. Thirteen had good or excellent results after loose or attached capitellar segment was removed and shaved to bleeding bone. This was done via a lateral arthrotomy, without an attempt at fixation. The lesions were not sized.
In 1992, Bauer et al29 reported average 23-year follow-up(range 11 to 35 years) on 31 patients. Eight were less than 16 years of age and 23 were older than 16 years. About half the elbows were symptomatic at follow-up. No stratification was done based on radiographic criteria. Only six patients had demarcated islands of bone, and 20 had loose bodies. Twenty-three of 31 were treated surgically, 19 for removal of loose bodies. Advanced lesions were seen in over half the cases. A 10-degree flexion and extension loss was noted. Twenty-seven of 31 had increased radial head diameter, and 19 of 31 had signs of degenerative joint disease.
In 1989’ Jackson et al reported 10 cases of OCD in female gymnasts. The average age was 12.5 years (range 8–17 years). Nine out of the 10 underwent curettage of loose articular cartilage and drilling of the bed and removal of loose bodies. Follow-up averaged 2.9 years (7 months to 7 years). Only one athlete returned to sports, but participated with discomfort. Average loss of extension was 9 degrees and flexion was 2 degrees. The authors felt that once radiographs are positive and nonoperative treatment fails, surgical intervention can improve symptoms, but return to gymnastics was unlikely.
Peiss et al40 in 1995 published their initial experience of three patients treated based on an MRI with intravenous gadopentetate-dimeglumine contrast. They felt enhancement of the lesion itself, as opposed to around the lesion, indicated blood supply to the lesion and hence viability. Their article is largely anecdotal, due to the small number of patients involved, but it does raise the question as to whether or not the viability of the fragment should be taken into account when deciding treatment.
Klekamp et al11 in 1997 published a series of seven cases with an average age of 13 years, in whom OCD of the humeral capitellum led to posterolateral rotatory instability. Treatment involved open reduction and internal fixation with 1.5-mm metal screws. At an average of 3.2 years follow-up, range of motion had improved in extension 17 degrees and the elbows were stable.
Janarv et al9 reported on 13 consecutive patients who underwent shaving and/or drilling of the OCD bed, and loose body removal was done in 11 of the 13. The patients averaged 13.5 years of age (range 11–16 years), with follow-up of 1.3 years. The lesions measured between 10 and 20 mm, were round, and located on the anterior inferior capitellum and radial head. Procedures were done both open and arthroscopically, and all 13 improved. Twelve returned to their desired level of activity; however, none were gymnasts and five of the 11 treated surgically participated with symptoms. Range of motion improved. The authors noted radial head OCD in combination with capitellar OCD lesions in three of the 13 patients, and one had isolated radial head OCD. MRI detected only two of the four patients with radial head involvement. Preoperative MRIs clearly demonstrated the pathologic cartilage on the capitellum confirmed at the time of surgery. No comment in regard to fragment size, stability, or viability was made. No intravenous contrast was used. In six patients, dorsally based flaps were noted and debrided. Flaps or loose bodies were noted in patients with symptoms of locking and indicated advanced disease.
Ruch et al6 reported on 12 patients, average age of 14.5 years, who underwent arthroscopic debridement alone. Follow-up was 3.2 years. Flexion contractures improved on average from 23 to 10 degrees. All had remodeling of the capitellum, with five of 12 demonstrating enlargement. Five patients had a triangular avulsion fragment off the lateral condyle that was seen radiographically. Interestingly, this was not demonstrable at arthroscopy but correlated with a poor outcome. Lesions ranged from 0.75 to 4.2 cm2 with an average square area of 2.5 cm2. Seven of 12 patients had detached lesions, whereas five had hinged lesions. All lesions were debrided. Total arc of elbow motion improved from 110 to 127 degrees. Flexion contractures improved, on average, from 23 to 11 degrees. All patients demonstrated remodeling of the capitellum.
Baumgarten et al5 reported an arthroscopic classification (Fig. 19–6) based on a review of 17 elbows (16 patients) with an average follow-up of 48 months (range 24–75 months). Average age was not reported. Their classification separated lesions into five categories and gave suggestions regarding treatment based on the grade of the lesion: Type 1 had intact chondral surface, and observation or drilling of lesion was advocated. Type 2 had fissuring of the articular cartilage identified by probing, and treatment involved resection back to stable cartilage. Type 3 had a fragment that was loose on probing, and the fragment was removed with the aid of an osteotome. Type 4 had a grossly loose fragment and was removed. Type 5 had an empty crater that was burred to bleeding bone, and the loose bodies were removed. Lesion size was not mentioned. At follow-up, the average flexion contracture improved from 19 to 5 degrees. Four of the 17 elbows had pain; however, no correlation with the lesion type was made. Two of the nine throwers and one of the five gymnasts did not return to sports. Eight of 17 demonstrated flattening of the capitellum but no degenerative joint disease at 48 months.
Recently, Byrd and Jones50 reported on 10 patients with 3.9-year follow-up using the above classification. The average age was 13.8 years (range 11–16 years) and were skeletally immature. All patients had pain as a presenting symptom. Loss of extension was more significant (18 degrees) than loss of flexion (11 degrees) when motion loss was a presenting symptom. Four of 10 had locking symptoms at presentation. Objective findings correlated poorly with stage of lesion. The OCD lesion was present on all x-rays. However, the size of the lesion was never reported. All patients were treated arthroscopically and included synovectomy, abrasion chondroplasty, and loose body removal depending on the nature of the individual lesion. Postoperative results were excellent. However, the follow-up was limited. All patients returned to some competitive sport but only 40% returned to their original sport.
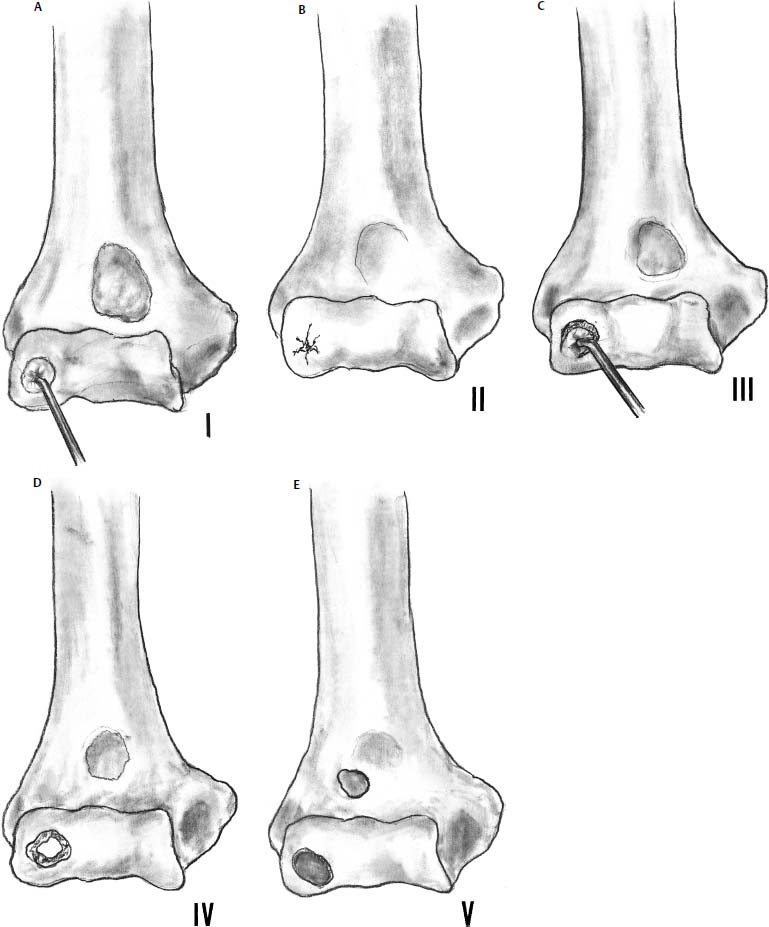
FIGURE 19–6 Arthroscopic classification of osteochondritis dissecans of the capitellum. (A) Type I: the cartilage is intact but is soft and ballotable. (B) Type II: fissuring of the cartilage. (C) Type III: exposed bone with a partially attached osteochondral flap. (D) Type IV: completely detached fragment but nondisplaced. (E) Type V: displaced fragment with a loose body. (Adapted from Baumgarten T, Andrews J, Satterwhite V. The arthroscopic classification and treatment of osteochondritis dissecans on the capitellum. Am J Sports Med 1998;26:520–523.)
To elucidate the early pathologic changes seen in OCD, Takahara et al15 in 1998 used MRI and ultrasound to diagnose very early OCD when radiographic changes were subtle. T1-weighted MRI demonstrated a low signal in the superficial aspect of the capitellum, with no demonstrable change on T2-weighted images. The authors felt that this represented the earliest changes seen in OCD of the capitellum. Cessation of pitching in two of the three patients resulted in diminution in symptoms and subsequently normal x-rays and elbow function. The third patient continued to throw and went on to develop classic OCD with a painful elbow. Takahara et al also reported a series of 15 patients, average age of 13.3 years (range 11–16 years) with an average of 5.2 years of follow-up. All patients were advised to stop the inciting trauma for 6 months. At follow-up 17% had no pain, 29% had mild pain with heavy activity, and 54% had pain with activities of daily living. Five of the 11 early lesions, classified by radiographs demonstrating rarefaction and flattening of subchondral bone, improved, demonstrating that there is a propensity to heal early lesions but in only 50% of the cases. All four of the advanced lesions showed no improvement radiographically. More recently, they reported a series of 53 patients, 39 of whom underwent operative and 14 of whom underwent nonoperative treatment.4 The average age was 16.6 with 12.6-year follow-up. Surgical treatment involved removal of loose bodies and debridement of the fragment. This is the first report to correlate lesion size with outcome. The defect was sized by percent defect size and defect angle (Fig. 19–7). A large defect measured >70% with a defect angle of 90 degrees. A small defect measured <55% with a defect angle of <60 degrees; all others were classified as moderate. The chronicity of the lesion had no value in predicting outcome as six of 19 early lesions (32%) and 13 of 26 late lesions (50%) had a poor outcome. No very early lesions were reported in this series. Predictors of a poor radiographic outcome included early degenerative joint disease in nine of 14 (64%) as opposed to 10 of 32 (31%) without degenerative joint disease. Defect size correlated with outcome; seven of seven large lesions, six of 19 moderate lesions, and zero of six small lesions had a poor outcome. According to Takahara et al, these results suggest that large lesions should be addressed. They recommended using drilling, reduction and fixation, allograft or autologous chondrocyte implantation.4,31,51–53
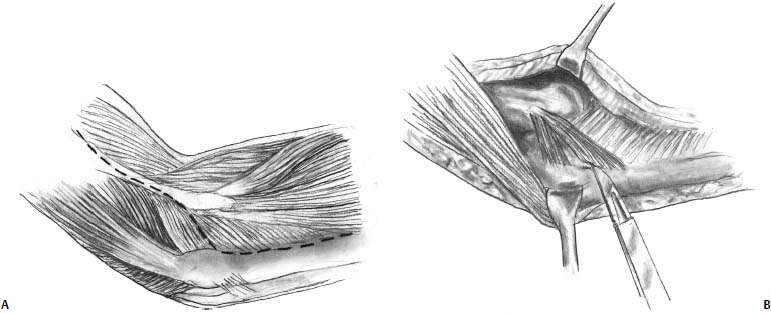
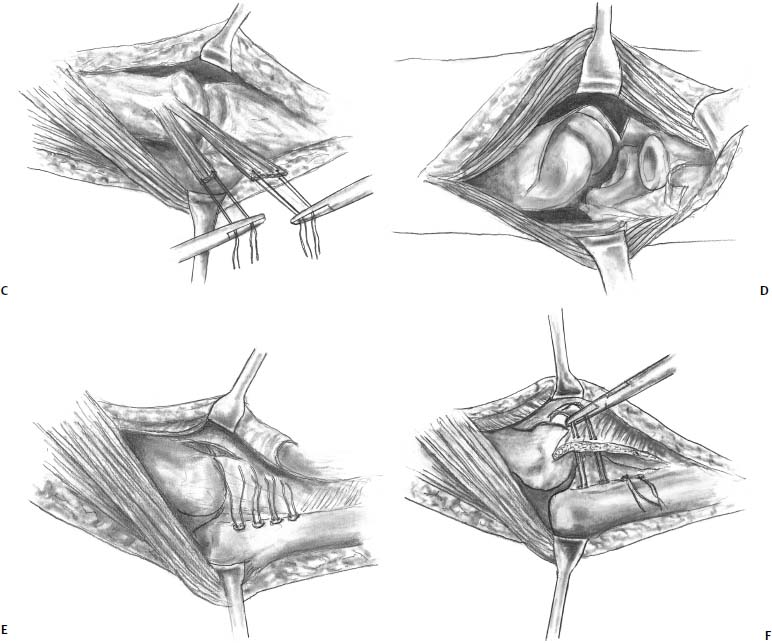
FIGURE 19–7 Posterior Boyd approach to the capitellum. (A) A posterior skin incision is used and deep incision is started from the lateral column and carried distally under the anconeus. (B) The lateral collateral ligament complex is then sharply elevated off of the ulna using a sharp blade or needle tip cautery. (C) Tagging sutures are placed through the ligament complex. (D) By releasing the lateral collateral ligament complex, the radiocapitellar joint can easily be opened allowing easy access to the capitellum. (E) Once the capitellar lesion is addressed, suture anchors are placed in the ulna along the crista supinatoris. (F) The sutures are passed through the ligament complex and repaired back down to the ulna.
Oka et al7 reported on bone peg grafting of an OCD fragment with autologous cortical bone taken from the proximal ulna at the time of open arthrotomy. They classified the lesions using a modification of the Minami classification: Type I lesions showed a translucent window in the lateral or middle capitellum. Type II lesions showed a clear zone or line between the OCD and the capitellar subchondral bone. Type III lesions demonstrated loose bodies. There were 32 patients from 4.8 to 20 years of age. Twenty-one of the 32 patients played baseball. Type I or II lesions occurred in 20 elbows and underwent grafting with two cortical bone pegs, measuring 3 × 3 × 30 mm. A pilot hole was drilled with a 2.5-mm Kirschner wire to a depth of 30 mm. Bone pegs were then tapped into place and sheared flush with the chondral surface. Ten elbows underwent nonoperative treatment initially, four of which later underwent bone peg grafting. Six elbows had a loose body only. Six of the 20 that had bone peg grafting also had loose body removal. Range of motion improved 7 degrees in patients who had bone peg grafting only, 15 degrees in patients with combined bone peg grafting and loose body removal, and 8 degrees with loose body removal only. Patients undergoing nonoperative treatment lost 2 degrees. Five of the 10 with nonoperative treatment healed similarly to the patents reported by Takahara et al. Four patients did not improve and subsequently underwent bone peg grafting. Fifteen of the 16 patients with bone pegs healed. Fifteen patients were followed for 5 to 10 years. Radiographs revealed osteoarthritis with spur formation. Bone pegs were used in two type I and six type II lesions. The bone peg group did much better but statistically this was not significant.
Takeda et al12 reported on a technique using curettage and local bone grafting with removable wire fixation for unstable OCD lesions in 11 skeletally immature baseball players. Average age was 14.7 years and follow-up averaged 57 months. Bony union averaged 17 weeks. Ten of the 11 patients returned to their previous level of activity. The procedure was done through an open lateral approach to the elbow, and required detaching the lateral ulnar collateral ligament complex from the lateral epicondyle. The wire was removed as an outpatient procedure under local anesthesia following bony union.
Harada et al13 reported on four patients treated with curettage and local bone grafting secured with a compression staple applied from the lateral aspect of the elbow. Follow-up averaged 7.5 years (range 2–11 years); three of the four were able to return to throwing without pain.
Sato et al14 recently published a case report on a modification of autologous chondrocyte transplantation described by Brittberg et al.54 Sato et al implanted a gel impregnated with chondrocytes under a periosteal patch. Follow-up arthroscopy was performed at 3 and 24 months. The patient was pain free and performing activities of daily living without restriction.
 Treatment Guidelines for Skeletally Immature Athletes
Treatment Guidelines for Skeletally Immature Athletes
The term skeletally immature is variable at a biologic level. It is a continuum, as adolescent athletes mature at different rates. For example, a 14-year-old female athlete who has not started menses is considerably less skeletally mature than another female athlete who has been menstruating for 1½ years despite open growth plates seen on radiographs. There can be big differences in the male population as well. Despite this, some attempt needs to be made to assess skeletal maturity. We have traditionally used radiographs, noting the presence or absence of open physes. But as noted above, the onset of menses as well as the development of secondary sexual characteristics are also helpful but difficult to factor into a treatment regimen. In general, we are less aggressive surgically with skeletally immature patients based on the assumption that there is a greater propensity to heal prior to physeal closure and skeletal maturity.
When treating a young athlete with elbow pain, one must have a high index of suspicion for OCD, particularly if he or she is a baseball pitcher or gymnast. Our approach to this problem is still evolving, as some controversy exists as to the best imaging modality and treatment. The following discussion is intended as a guide, not as the only approach to OCD of the elbow in the immature athlete.
We recommend obtaining anterior-posterior and lateral radiographs of the elbow. Negative radiographs or radiographs with very subtle findings warrant 45-degree flexion anterior-posterior and contralateral views, paying close attention to subtle rarefaction and flattening of the capitellum.15 In the high-risk athlete, such as a pitcher or gymnast, we obtain an MRI. The use of intravenous and intraarticular contrast depends largely on radiographic findings and symptoms.
Traditional treatment guidelines are based on whether the lesion is intact, partially attached, or completely detached, and is based primarily on radiographic evaluation.26 We agree with this approach. Information obtained from MRI and in particular intravenous and intraarticular contrast can help guide treatment as these modalities have the potential to pick up unstable lesions that might otherwise be considered stable on conventional radiographs. However, they have not been shown in the literature to have prognostic value, as the reports using these modalities have limited numbers.15,40 Nevertheless, we feel they are beneficial as they provide information not obtained using conventional radiographs.
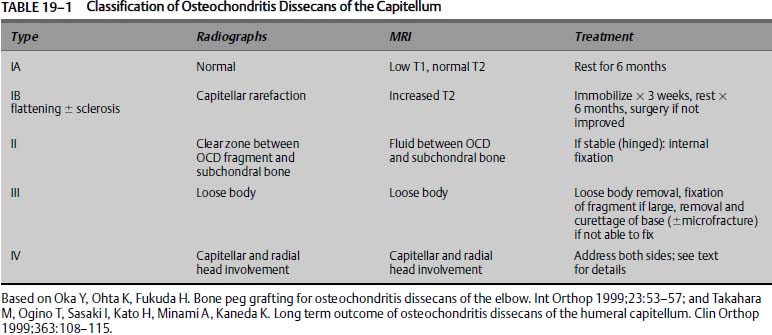
We have expanded the traditional classification to include subtyping of type I lesions, and we added a type IV lesion (Table 19–1). Takahara’s work suggests that with the advent of MRI, there is a subset of early lesions, with essentially normal or nearly normal radiographs, that can be identified by MRI or ultrasound early enough so that nonoperative treatment has a better prognosis.15 For this reason we divide type I lesions into A and B subtypes. Type IA lesions represent those lesions with essentially normal radiographs and MRI findings of low signal on T1-weighted images and normal T2-weighted images consistent with very early OCD. Type IB lesions have the more classic radiographic findings of early OCD with capitellar rarefaction, flattening, or sclerosis, and MRI findings of classic OCD with increased signal on T2-weighted images. The recognition of associated radial head lesions warrants, in our opinion, expanding the traditional classification to take into account these “bipolar” lesions.9 Because this adds a dimension of difficulty in treating patients and likely represents a more advanced lesion, we designated it a type IV lesion. This modification of the traditional classification has been presented elsewhere.55
Treatment should take into account many variables, including skeletal maturity, size of lesion, the stability or presumed stability, viability of the fragment, the presence or absence of mechanical symptoms, and response to nonoperative treatment. Lastly, the precise surgical intervention will be made on the merits of the capitellum and overlying articular cartilage seen at surgery. No one piece of information or test can be used to make treatment decisions. The following discussion is a guideline for treatment and has been put in table and algorithm form (Table 19–1 and Fig. 19–8). This does not represent the only approach to this problem.
Type IA: Very Early Lesions
These lesions have essentially normal or near normal radiographs. The diagnosis is confirmed by MRI, which demonstrates a low signal in the superficial capitellum on T1-weighted images and normal T2-weighted images.15 If an OCD is picked up at this point, we feel that it represents a very early lesion and is therefore stable and the capitellum is viable. Contrast is unlikely to be helpful as the capitellar viability is high. This subset is likely to do well with nonoperative treatment alone. A near-normal joint may result. Treatment includes activity modification, that is, complete cessation of the inciting trauma. Strengthening is begun once symptoms have abated. The athlete is not returned to sports for at least 6 months and then only if symptoms have completely resolved. Follow-up radiographs at 3 and 6 months are obtained to assess progression. Follow-up may be required for a period of years. Pitchers are counseled to stop pitching but may return to another fielding position. Gymnastics is more difficult as the elbow becomes a weight-bearing joint, and there is no option to return to a modified program and remain highly competitive. Return to sports is predicated on symptoms, as radiographic changes may be present for years.3,15,26 We monitor these patients very carefully. Return of symptoms warrants additional time off from sports. Patients with a normal T1-weighted MRI scan do not return to sports activities until resolution of symptoms, as the diagnosis is likely a soft tissue overuse injury, such as lateral epicondylitis.
Type IB: Early Lesions
Athletes with early lesions demonstrate the more classic early findings of OCD on radiographs, such as capitellar rarefaction, mild flattening, and sclerosis. Once obvious radiographic findings are apparent, we feel it is important to assess the stability and viability of the fragment. Type IB lesions are at risk for articular cartilage breakdown and fragment instability. Mechanical symptoms, however, are notably absent. Therefore, these patients also undergo an MRI. A conventional MRI is adequate for assessing subchondral cysts and fluid, which would indicate an unstable lesion. However, it is in this setting that intravenous contrast and intraarticular contrast have potentially the most benefit. Although neither is mandatory, we have traditionally favored the use of intraarticular contrast. Dye leaking between the fragment and adjacent subchondral bone suggests an unstable lesion and hence a break in the articular cartilage. However, an argument can be made for using intravenous contrast, in which case enhancement as a halo around the fragment suggests perifragment scarring and hence instability of the lesion. Enhancement of the lesion itself suggests viability. In the situation that the MRI demonstrates no instability and viability of the fragment, the arm is immobilized for no more than 3 weeks to allow any acute symptoms to resolve. Following this, we institute the aforementioned physical therapy with the initial goal of obtaining full range of motion. Patients are followed clinically, and radiographs are obtained at 3-month intervals to evaluate progression of healing. Return to play is predicated on resolution of symptoms, but a minimum of 6 months of rest is instituted. Patients are counseled as to the long-term implications of this problem and the unpredictability of nonoperative treatment.
Patients who have failed 6 months of nonoperative treatment, as defined by persistent symptoms of pain, or who have demonstrable instability of the OCD fragment on MRI, undergo surgical intervention. We recommend arthroscopy first, and if necessary, conversion to an open procedure through an anterolateral approach. The actual procedure performed and the method by which it is done, that is, open or arthroscopic, depend on the nature of the lesion and the surgeon’s skill. Very small lesions will probably do well, irrespective of the treatment. Lesions measuring less than 55% of the capitellum with less than a 60-degree defect angle should undergo subchondral drilling as described by Bradley and Dandy.31 Damaged articular cartilage should be debrided to bone with stable articular cartilage edges. We do not recommend the use of thermal techniques as recent evidence suggests this may be detrimental to remaining chondrocytes (James P. Bradley, M.D., personal communication).
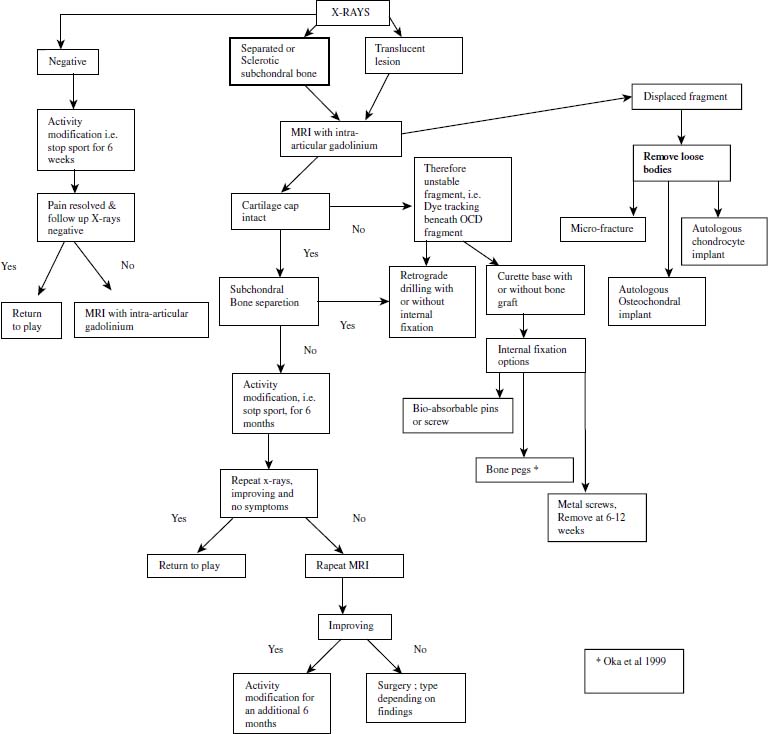
FIGURE 19–8 Treatment algorithm for OCD of the elbow.
In medium-sized defects, the status of the overlying articular cartilage and the integrity of the underlying bone determine the treatment. We attempt to internally fix acute lesions if possible. Chronic defects with fragmentation are debrided. Subchondral drilling or microfracture may also be performed. Internal fixation can be considered in some of these lesions with a single fragment. Acceptable choices include metal screws, bioabsorbable screws, cortical bone pegs, and pull-out wires. Machined allograft cortical screws are now available and raise the possibility of compression with biointegration. We have no experience using these devices. We prefer metal or bioabsorbable implants; however, concern over the absorption of bioabsorbable devices across an area with unequal blood flow has limited their use, along with the difficulty in obtaining compression. Osteochondral autograft transplantation is considered. The relative risks and benefits make it difficult to make a definitive recommendation in this population. It is reasonable to consider a two-staged treatment program based on clinical follow-up. If patients continue to have symptoms at 6 to 9 months following debridement and subchondral drilling or microfracture, then consideration is given to additional intervention in the form of osteochondral autograft or autologous chondrocyte implantation.
Large defects measuring greater than 70% of the anterior-posterior capitellar dimension and greater than 90 degrees on the lateral have a poorer long-term outcome.4,15 With these large defects particularly with subchondral bone loss, we attempt to reconstitute the capitellum. In some cases, a large fragment remains hinged and attached. The sclerotic bone bed is debrided with a curette. This is done in an open fashion. Autograft cancellous bone is obtained from the ulna and packed into the defect. Metal compression screws are used to secure the fragment. The patient is followed with serial radiographs to ensure reformation of subchondral bone. Removable wires can also be used.12 Screws are removed 3 to 5 months later. Alternatively, bioabsorbable screws can be used, in which case removal is typically not necessary. However, obtaining compression can be difficult. Headless variable pitch metal screws are available and allow for compression, and they represent another option, although we have not used them. Comminuted fragmented OCD lesions are debrided. Immediate consideration is given to autograft osteochondral transplantation, particularly if significant subchondral bone loss is present. Recently, synthetic grafts (Osteobiologics Inc., San Antonio, TX) have been developed for bone and articular cartilage, raising the possibility of addressing these lesions without donor-site morbidity. We have no experience using these grafts and no clinical studies have been published, but the animal studies are encouraging. This procedure is done in an open fashion. It is difficult to obtain congruency of the joint. However, it must be kept in mind that this is a salvage situation. We have not used allograft, autologous chondrocyte implantation,54 or tissue engineered cartilage,14 although these remain viable alternatives.
Surgical Technique
Open Technique
The patient is placed in a supine position. A lateral incision is made and the Kocher interval is entered. [Editor’s note: The book editor uses the posterior Boyd56 approach to gain access to the capitellum (Fig. 19–7).] Either a lateral or a posterior incision is made. The deeper incision is started from the lateral column proximally and taken distally posterior to (under) the anconeus. This is different from the Kocher approach, which enters the interval between the anconeus and extensor carpi ulnaris. Next, the insertion of the lateral collateral ligament complex onto the ulna, including the annular ligament, is subperiosteally elevated off of bone (crista supinatoris). Tagging sutures are placed in the ligament for future repair back down to bone. By the surgeon’s supinating the patient’s forearm and flexing the elbow, the radial head can be subluxated laterally, facilitating access to the capitellum. At this point, either an osteochondral transfer or an autologous chondrocyte implantation procedure can be performed. Once the procedure is completed, three to four suture anchors are placed into the ulna, and the lateral collateral ligament is repaired back down to the ulna. [The book editor prefers 3.5-mm metal corkscrew anchors loaded with No. 2 Fiberwire suture (Arthrex, Naples, FL).]
Type II
These lesions are distinguished from type IB lesions by more advanced changes on x-ray and in particular a sclerotic margin around a well-defined fragment. In reality this distinction is extremely difficult, and the two lesions are approached in essentially the same manner. Mechanical symptoms may be present from a loose attached lesion. They are distinguished from type III lesions by the lack of loose bodies. Similar to the management of type IB lesions, an MRI is obtained. In our opinion type II lesions are more likely to be unstable and lack viability. We favor intravenous contrast or intraarticular contrast over conventional MRI. Patients with mechanical symptoms or lesions demonstrating instability on MRI are in our opinion unlikely to heal. We are surgically more aggressive with this population of patients. Surgical intervention is essentially the same as that employed for type IB lesions. Hinged fragments are left attached if large enough to accept internal fixation. The subchondral bed is curetted and bone grafted usually from the proximal ulna. Internal fixation is then used. We recommend routinely removing headed metal screws. Enhancing lesions with evidence of instability have not been encountered in our experience, neither has it been reported in the literature.
Type III Chronic Lesions with Loose Bodies
The presence of loose bodies usually indicates a longstanding lesion, although acute dislodging of an in situ lesion can occur. Diagnostic workup proceeds in the same manner as for types IB and II lesions. Intraarticular contrast is helpful in detecting loose bodies and as such may have benefit over intravenous contrast. Most authors agree that removal of loose bodies is indicated, and there is no role for reduction and internal fixation. There is no consensus regarding reduction and internal fixation of an acutely dislodged fragment. However, we would favor fixation depending on the size, location, and integrity of the fragment and if the acuteness of the injury could be well established. Chronic loose bodies are removed. Treatment of the capitellar lesion is based on the principles of treating unstable types IB and II lesions. Patients and family should be counseled appropriately as to the expected outcome. We do not recommend returning patients to sports.
Type IV Associated Radial Head Osteochondral Defects
Radial head lesions occasionally accompany the more common capitellar lesions.9 We have not had the occasion to treat this combination of injury. As in the knee, this represents a bipolar lesion, and it makes treatment more difficult. Obtaining access to the face of the radial heads is difficult without dislocating the elbow. The value of reconstituting the articular surface of the capitellum in this setting is unknown. If the radial head lesion is small, we would favor treating the capitellar lesion. There is no consensus as to what “small” constitutes, but less than 30% of the articular surface is probably reasonable. With large degenerative bipolar lesions, simple debridement, curettage, and subchondral drilling or microfracture is likely to be more prudent, although we have no experience in this setting. In those patients with severe advanced degenerative changes at the radiocapitellar joint, particularly in adulthood, consideration is given to radial head excision.
 Treatment Guidelines for Skeletally Mature Athletes
Treatment Guidelines for Skeletally Mature Athletes
Our approach is essentially the same in the skeletally mature athlete. However, once early degenerative changes occur, we advise our patients that early arthritis is likely to occur. Nonoperative treatment is less likely to be effective, and surgical intervention is consider early in the treatment regimen.
Our approach to the focal lesion attempts to take into account the size and depth location and acuteness of the lesion. Most of these lesions are chronic, and thus open reduction and internal fixation of the acute dislodged OCD is not done. We try to stair-step the approach beginning with subchondral drilling or microfracture for small lesions. We avoid abrasion chondroplasty in an attempt to maintain some of the subchondral plate if possible. With medium-size and large lesions, consideration is given to osteochondral transplantation using fresh-frozen allograft or autograft from the lateral aspect of the ipsilateral knee. Autologous chondrocyte implantation (ACI) can certainly be considered, but we have no experience with this procedure in the elbow.
 Future Directions
Future Directions
There are several current controversies in the diagnosis and treatment of OCD of the humeral capitellum. The use of intravenous or intraarticular gadolinium to assess fragment viability and stability remains a subject of debate. We have no experience with intravenous gadolinium. Currently, intraarticular contrast is favored in the United States. Traditional treatment consists primarily of removal of loose bodies and debridement of the lesion.26 This approach is supported by the literature. However, most of the studies this approach is based on have not used newer diagnostic or treatment modalities. More research needs to be done to determine whether accurate prognostic data can be obtained from intravenous contrast as it relates to fragment viability. Just because a fragment enhances does this mean it is viable? Can it be treated nonoperatively? Or is there another variable such as size in conjunction with gadolinium enhancement that should dictate treatment?
Moreover, once operative treatment has been selected what factors should dictate the procedure used? If fixation is selected should metal, bone, or a bioabsorbable device be used? Each has a particular advantage and disadvantage. We have concerns regarding the use of bioabsorbable devices for articular cartilage injuries. In theory degradation could occur from proximal to distal. When the humeral aspect of the screw has resorbed, the OCD part of the screw may still be present. As such there is the potential for developing an intraarticular loose body or simply loosening of the fragment. To our knowledge, this issue has not been addressed by the manufacturers producing these devices, nor has it been addressed in the orthopaedic literature. This concern may be overstated, as most of the bioabsorbable devices are degraded by hydrolysis and should degrade in synovial fluid. Recently, however, MRI evidence has surfaced corroborating this potential pitfall in the knee, although it remains anecdotal (Dr. Christopher Harner, personal communication). Cortical bone pegs provide an alternative that would allow all biologic fixation presumably without the need for removal.7 No compression can be obtained with this method, and additional dissection is needed to harvest the grafts. However, with the anatomy of the elbow, compression may not be a major issue as muscle forces acting through the radial head may provide enough compression. Cortical screws machined from cadaveric cortical bone can potentially provide compression and allow for biointegration as they incorporate as bone. Such screws would have to be advanced to at least the level of the OCD fragment calcified cartilage layer. They are, however, expensive, and some surgeons believe the use of allograft tissue in adolescents is undesirable. Furthermore, the screws do not have a variable pitch, so the amount of compression is likely to be minimal, and they are headed screws similar to traditional screw designs and have the potential to be prominent. The distinction between bioabsorption and biointegration is a subtle but potentially important point that may direct future research. The ideal fixation device is one that incorporates into bone, allows for compression, and is inexpensive.
The location and presence of bipolar lesions are largely ignored in most series. Does having an associated radial head OCD affect outcome? And if so, what is the ideal treatment in this setting?
The future of treating established lesions with some osteochondral replacement remains the objective of operative intervention. Sellers et al57 improved treatment of articular cartilage defects with a recombinant human bone morphogenic protein-2 (rh-BMP-2)-impregnated collagen sponge in New Zealand white rabbits. One year follow-up demonstrated that the repair process held up with time and had improved the graft-host cartilage interface, one of the most difficult areas to address. Potential future options include chondrocyte-impregnated collagen gels. Recently synthetic grafts have been developed to address both bone and articular cartilage defects. These grafts are composed of various polymers (Osteobiologics Inc., San Antonio, TX) may prove beneficial in the treatment of OCD by avoiding the morbidity associated with osteochondral transplant procedures.
 Conclusion
Conclusion
Osteochondritis dissecans of the humeral capitellum remains a difficult problem to treat. Controversy still exists with respect to the optimal treatment of loose in situ lesions (i.e., loose but attached). Rest is the treatment of choice for very early lesions, and studies suggest that if diagnosed early enough, a near-normal elbow can result.15 However, early detection and intervention remain difficult as early OCD may or may not present with pain and discomfort significant enough for the athlete to seek medical attention early enough in the process where intervention has a predictably positive outcome. Nevertheless, elbow pain in the at-risk athlete, such as a baseball pitcher or gymnast, should raise the suspicion of an OCD. Radiographs may be unrevealing or show very subtle changes. Contralateral elbow views are very helpful. The advent of MRI now allows the practicing orthopaedic surgeon to effectively assess very early lesions that might otherwise be missed on x-ray.
With more advanced lesions, x-ray findings are more obvious and demonstrate the more classical capitellar fragment with a surrounding zone of lucency. MRI in this setting is helpful in assessing the overlying articular cartilage and hence the stability of the fragment. In this setting, prior to obvious loose bodies or mechanical symptoms, rest is the first step in the treatment algorithm. If symptoms persist, then operative intervention is indicated. About half of these patients heal with nonoperative treatment. Pretreatment assessment of fragment viability has not traditionally been incorporated into the treatment algorithm. Recent anecdotal evidence suggests that not only stability but viability of a fragment can be assessed using intravenous contrast. Knowledge of the fragment viability may allow distinction between those lesions likely to heal without surgical intervention versus those requiring surgical intervention.
Most authors would agree that there is no role currently for reduction and fixation of long-standing free loose bodies. There is no consensus regarding acute dislodging of an in situ loose fragment.
Long-term results, after radiographic changes are present, suggest a degenerative course in about half the patients. Whether or not newer techniques to address osteochondral defects will have an effect on the natural history remains to be seen.
REFERENCES
1. Paré A. Oeuvres Completes, vol. 3. Paris J.B. Ballière, 1840–1841:32
2. Jackson D, Silvino N, Reimen P. Osteochondritis in the female gymnast’s elbow. Arthroscopy 1989;5:129–136
3. Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med 1999;27:728–732
4. Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop 1999;363:108–115
5. Baumgarten T, Andrews J, Satterwhite V. The arthroscopic classification and treatment of osteochondritis dissecans on the capitellum. Am J Sports Med 1998;26:520–523
6. Ruch D, Cory J, Poehling G. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy 1998;14:797–803
7. Oka Y, Ohta K, Fukuda H. Bone peg grafting for osteochondritis dissecans of the elbow. Int Orthop 1999;23:53–57
8. McManama GB Jr, Micheli LJ, Berry MV, Sohn RS. The surgical treatment of osteochondritis of the capitellum. Am J Sports Med 1985;13:11–21
9. Janarv P-M, Hesser U, Hirsch G. Osteochondral lesions in the radiocapitellar joint in the skeletally immature: radiographic, MRI, and arthroscopic findings in 13 consecutive cases. J Pediatr Orthop 1997;17:311–314
10. Inoue G. Bilateral osteochondritis dissecans of the elbow treated by Herbert screw fixation. Br J Sports Med 1991;25:142–144
11. Klekamp J, Green N, Mencio G. Osteochondritis dissecans as a cause of developmental dislocation of the radial head. Clin Orthop Relat Res 1997;338:36–41
12. Takeda H, Watari K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent players. Am J Sports Med 2002;30:713–717
13. Harada M, Ogino T, Takahara M, Ishigaki D, Kashiwa H, Kanauchi Y. Fragment fixation with a bone graft and dynamic staples for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg 2002;11:368–372
14. Sato M, Ochi M, Uchio Y, Agung M, Baba H. Transplantation of tissue-engineered cartilage for excessive osteochondritis dissecans of the elbow. J Shoulder Elbow Surg 2004;13:221–225
15. Takahara M, Shundo M, Kondo M, Suzuki K, Nambu T, Ogino T. Early detection of osteochondritis dissecans of the capitellum in young baseball players. Report of three cases. J Bone Joint Surg 1998;80-A:892–897
16. Vanthournout I, Rudelli A, Valenti P, Montagne JP. Osteochondritis of the trochlea of the humerus. Pediatr Radiol 1991;21: 600–601
17. Chess T. Osteochondritis. In: Savoie F, ed. Arthroscopy of the Elbow. New York: Churchill-Livingstone, 1996:77–86
18. Bednarz P, Paletta GJ, Stanitski C. Bilateral osteochondritis dissecans of the knee and elbow. Orthopedics 1998;21:716–717
19. Peterson R, Savoie F, Field L. Osteochondritis dissecans of the elbow. Instr Couse Lecture 1999;48:393–398
20. Schenck R. Current concepts review: osteochondritis dissecans. J Bone Joint Surg 1996;78-A:439–455
21. König F. Ueber freie köper in den gelenken. Deutsche Zeitchr Chir 1887;27:90–109
22. Schenck RJ, Goodnight J. Osteochondritis dissecans. J Bone Joint Surg 1996;78-A:439–456
23. Nagura S. The so-called osteochondritis dissecans of König. Clin Orthop 1960;18:100–122
24. Panner HJ. A peculiar affection of the capitellum humeri, resembling Calve-Perthes disease of the hip. Acta Radiol 1927;8:617–618
25. Omer GE Jr. Primary articular osteochondroses. Clin Orthop Relat Res 1981;158:33–40
26. Shaughnessy W. Osteochondritis dissecans. In: Morrey B, ed. The Elbow and Its Disorders. Philadelphia: W.B. Saunders, 2000: 255–260
27. Vispo-Seara J, Loehr JF, Krauspe R, Gomlke F, Eulert J. Osteochondritis dissecans in children and adolescents. J Shoulder Elbow Surg 1995;4:S21
28. Holland P, Davies A, Cassar-Pulucino V. Computerized tomographic arthrography in the assessment of OCD of the elbow. Clin Radiol 1994;49:231–235
29. Bauer M, Jonsson K, Josefsson PO, Linden B. Osteochondritis dissecans of the elbow. A long term followup study. Clin Orthop Relat Res 1992;284:156–160
30. Schenck RJ, Athanasiou KA, Constantinides G, Gomez E. A biomechanical analysis of articular cartilage of the human elbow and potential relationship to osteochondritis dissecans. Clin Orthop Relat Res 1994;299:305–312
31. Bradley J, Dandy D. Results of drilling osteochondritis skeletal maturity. J Bone Joint Surg 1989;71-B:642–644
32. Hunter S. Little League elbow. In: Zarins B, Andrews J, Carson W, eds. Injuries to the Throwing Arm. Philadelphia: W.B. Saunders, 1985
33. Jobe F, Nuber G. Throwing injuries of the elbow. Clin Sports Med 1986;5:621–636
34. Indelicato P, Jobe F, Kerlin R. Correctable elbow lesions in professional baseball players. Am J Sports Med 1979;7:72–79
35. King J, Brelsford H, Tullos H. Analysis of the pitching arm of the professional baseball pitcher. Clin Ortho Relat Res1969;67: 116–123
36. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop RelatRes 1972;88:169–182
37. Tullos H, King J. Lesions of the pitching are in adolescents. JAMA 1972;220:264–271
38. Singer K, Roy S. Osteochondrosis of the humeral capitellum. Am J Sports Med 1984;12:351–360
39. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasulature in the distal humersu. Acta Orthop Scand 1959;38(suppl):1–232
40. Peiss J, Adam G, Casser R, Urhahn R, Gunther RW. Gadopentetate-dimeglumine-enhanced MRI imaging of osteonecrosis osteochondritis dissecans of the elbow: initial and experience. Skeletal Radiol 1995;24:17–20
41. Minami M, Nakashita K, Ishii S, Usui M, Muramatsu I. Twenty-five cases of osteochondritis dissecans of the elbow. Rinsho Seikei Geka 1979;14:805–810
42. Bradley J. Upper extremity: elbow injuries in children and adolescents. In: Stanitski C, De Lee J, Drez DJ, eds. Orthopaedic Sports Medicine Practice and Principles. Philadelphia: W.B. Saunders, 1994:242–261
43. Bunnell DH, Fisher DA, Bassett LW, Gold RH, Ellman H. Elbow joint: normal anatomy on MR images. Radiology 1987;165:527
44. Fritz R, Steinbach L. Magnetic resonance imaging of the musculoskeletal system. Part 3. The elbow. Clin Orthop Relat Res 1996;324:321–339
45. Fritz RC. MR imaging of osteochondral and articular lesions. MRI Clin North Am 1997;5:579–602
46. Middleton W, Macrander S, Kneeland JB, Froncisz W, Jesmanowicz A, Hyde JS. MR imaging of the normal elbow: anatomic correlations. Am J Radiol 1987;149:543–547
47. Steinbach LS, Fritz RC, Tirman PF, Uffman M. Magnetic resonance imaging of the elbow. Eur J Radiol 1997;25:223–241
48. Kramer J, Stiglbauer R, Engel A. MR Contrast (MRA) in osteochondritis dissecans. J Comput Assist Tomogr 1992;16: 254–260
49. Rosenberg Z, Beltran J, Cheng Y. Pseudodefect of the capitellum: potential MR imaging pitfall. Radiology 1994;191:821–823
50. Byrd JWT, Jones K. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players. Am J Sports Med 2002;30:474–478
51. Cugat R, Garua M, Cuscuo X. Osteochondritis dissecans: a historical review and its treatment with cannulated screws. Arthroscopy 1993;9:675–684
52. Brittberg M, Lindahl A, Nilsson A. Treatment of deep cartilage defects in the knee with autologous chondrocyte implantation. N Engl J Med 1994;331:889–895
53. Caplan A, Elyaderani M, Mochizuki Y. Principles of cartilage repair and regeneration. Clin Orthop Relat Res 1997; 342: 254–269
54. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defect in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331: 889–895
55. Bradley J, Petrie R. Osteochondritis dissecans of the humeral capitellum. In: Miller M, ed. Clinics in Sports Medicine. New York: W.B. Saunders, 2002
56. Boyd HB. Surgical exposure of the ulna and proximal third of the radius through one incision. Surg Gynecol Obstet 1940;71:86–88
57. Sellers RS, Zhang R, Glasson SS, et al. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (RHBMP-2). J Bone Joint Surg 2000;82-A:151–160
< div class='tao-gold-member'>



