I. PREPARATION FOR SURGERY
A. Scheduling surgery
- Prepare the patient so that the risks, goals, and benefits of the selected procedure are understood. The patient or legal next of kin should know the nature of the patient’s condition, the nature of the proposed treatment, the alternative treatments, the anesthetic risks, the anticipated probability for success, and the possible risks. Explain the postoperative dressings, casts or splints, exercise program, and other special requirements. When the patient has been so informed and has all questions answered, obtain a signed operative permit and mark the surgical sites with your initials.
- Review the technique of the proposed operation. At the time surgery is scheduled, be confident that the patient’s condition meets the appropriate indications for the proposed surgery. Know the anatomy and the surgical approaches involved in the selected surgical procedure. Carefully plan the procedure with the proper alternatives to reduce the length of time the wound is open. Be sure that all special equipment, implants, assistance, and time are available as expected. Complete any necessary templating of roentgenograms and preoperative planning drawings.1
B. Before surgery
- Patient preparation. Check to make sure the physical examination, chest roentgenogram, electrocardiogram, hemoglobin/hematocrit, and other indicated preoperative studies do not contraindicate surgery. Obtain a preoperative consultation from a specialist in internal medicine for all patients with unstable medical conditions. Many centers require preoperative clearance for patients over the age of 50 years. Order blood or type and screen, tetanus prophylaxis, and special medications as indicated. If an extremity operation is planned, be sure that the nails are properly trimmed and cleaned. Have the patient, family, and support system begin planning early for postdischarge or postoperation disposition needs, such as transportation home, wheelchairs, hospital beds, wheelchair access to the home, and commodes.
- Antibiotics2
a. Preoperative antibiotics should be administered for surgery that is associated with a high risk of postoperative deep wound infection, that is, when any implant is inserted, the operation results in a hematoma or dead space, the anticipated operating time is greater than 2 hours, or the surgeon is operating on bones, joints, nerves, or tendons.3,4 Various studies have shown immediate preoperative and postoperative antibiotics to be beneficial with surgery involving musculoskeletal tissues.2,4–6 See Chapter 4 for utilization of antibiotics with open wounds. The duration of antibiotic therapy can be limited to 24 hours postoperatively without increasing the risk to infection.
b. The timing of the antibiotic therapy is as important as dosage. Ideally, the antibiotic level should be highest when the tourniquet is inflated or the surgical hematoma (potential culture medium) is formed. Thus, the antibiotics must be given before surgery within 30 minutes of the incision. Because the highest blood levels with intravenous (IV) administration are achieved immediately, the ideal time to give IV antibiotics is when the patient is in the preoperative area or operating room during the 10- to 15-minute period just before the tourniquet is inflated or before the surgical incision is made. The antibiotics are readministered at the recommended intervals throughout the operative procedure (generally every 3 to 4 hours). The surgeon must also be aware of the effect of blood loss on the antibiotic levels. If the blood loss equals one half of the patient’s volume, approximately one half of the effective amount of the antibiotics has also been lost. The interval between the recommended doses for that patient, therefore, must be cut into half.
c. The authors recommend using one of the first-generation cephalosporins, which are bactericidal for bacteria usually found in wound infections following musculoskeletal surgery: staphylococcal and streptococcal specimens. The recommended antibiotics are listed in Table 11-1.
3. Patients who have been on long-term steroid therapy may need adjustments made in their steroid dosage when they undergo surgery or other major stress. The following is the simplest published regimen that the authors have found.7 The hospital service should be consulted to confirm the dosage plan:
a. On the day of surgery, order hydrocortisone sodium succinate (Solu-Cortef), 100 mg IV, to be given with the premedication before surgery.
b. Use the same dose on the first postoperative day.
c. Use 50 mg of hydrocortisone on the second postoperative day.
d. Use 25 mg of hydrocortisone on the third postoperative day, and then continue only with the patient’s normal oral daily dose.
4. Surgery in patients with insulin-dependent diabetes mellitus
a. In the morning before surgery, the patient should omit breakfast and take about one half of the normal insulin dose subcutaneously (SQ). Again, the hospital service should be consulted before final orders are placed.
b. After surgery, use a glucose measuring instrument every 4 to 6 hours to monitor blood glucose levels. The following sliding scale is useful: If the glucose level is greater than 350 mg per dL, give 15 units regular insulin SQ. If the level exceeds 250 mg per dL, give 10 units regular insulin SQ.
c. Return patients to their usual insulin dosage regimen as soon as they return to their normal activity level and to their usual American Diabetic Association diet.
5. Surgery in patients with hemophilia. Medical management of a patient with hemophilia who needs surgery requires precise assays of factor levels and prior survival studies of replacement factors to learn the effect of inhibitors and the biologic half-life in a particular patient. Aim to achieve 100% plasma levels just before anesthetics for surgery are administered. Maintain the level at 60% of normal for the first 4 days and more than 40% for the next 4 days. A level of 100% is also necessary for manipulation of a joint under anesthesia and for removal of pins. A 40% level is needed for suture removal. Levels of 20% are maintained for postoperative physical therapy for as long as 4 to 6 weeks after major joint surgery. Forty units of factor per kilogram of body weight administered just before anesthesia (unless survival studies done before surgery show that higher doses are needed) usually achieve close to 100% plasma factor levels. The hematology service should be contacted for assistance before placing the final orders.
C. Day of surgery
- Be sure the anesthesia technique proposed is adequate in terms of duration, muscle relaxation, and ability to position the patient properly.8,9 Supervise positioning, preparing, and draping so that the planned procedure could be accomplished without difficulty.10 Although the assistant prepares the patient, the surgeon can go to the instrument table with the scrub nurse and review major instruments required and implant from start to finish, outlining the planned procedure. The surgeon can also indicate what may be needed if any complications arise. The idea is to ensure that all equipment is immediately available, to review the procedure in the surgeon’s mind, and to prepare the entire surgical team so that the team and surgeon can work together efficiently. See Appendix E for the position and draping of the patient. See I.C.4.c for a discussion of skin preparations.
- Pneumatic tourniquets11–13
a. When a tourniquet is to be used, the necessary apparatus includes a cuff with a smooth, wrinkle-free surface that is a proper size. Select a tourniquet so that the width of the cuff covers approximately one-third of the patient’s arm length. Check the tubing for leaks. The tourniquet machine should have a safety valve release/alarm because excessively high pressures can cause paralysis. The inflating device must allow rapid attainment of desired pressure.
b. Plan surgery to minimize the operative time and, as a consequence, the tourniquet time.14 The conventional safe maximum inflation time of the tourniquet is 2 hours. The cuff may be applied about the arm or thigh but generally not about the forearm. There is no evidence that padding between the cuff and the skin is of any value, and such padding can cause skin wrinkles. Apply a plastic sheet with the adhesive edge placed on the skin distal to the tourniquet and cover the tourniquet with the plastic sheet as shown in Fig. 11-1, thereby preventing skin preparation solutions from getting underneath the cuff. Exsanguinate the limb with an Esmarch rubber bandage or with elevation of the limb above the patient’s heart for 60 seconds before inflating the tourniquet. An Esmarch bandage should not be used in cases of tumors or infection. Flexing the knee or elbow before inflating the tourniquet makes positioning and closure easier and prevents the possible complication of a ruptured muscle, which can occur by forced flexion of a tourniquet-fixed muscle. Rapidly inflate to the desired pressure. This is 175 to 250 mm Hg in the upper extremity, depending on the arm circumference and the patient’s systolic blood pressure, and 250 to 350 mm Hg in the lower extremity, depending on thigh circumference.11,15 Tissue pressure is always somewhat lower than tourniquet pressure, but at 30-cm circumference, it is close to 100%, declining to 70% at 60 cm circumference.11,15–17 The pressures should be decreased for infants and small children. Immediately after deflation, remove or loosen the cuff to prevent a venous congestion from proximal constriction of the extremity. If the tourniquet is deflated and reinflated during surgery, the time for reversal of the tourniquet-produced ischemia is proportional to the tourniquet time; that is, approximately 20 minutes is required for reversal after 2 hours of tourniquet time. In addition, tourniquet effects occur more rapidly after repeated use, and there is probably some summation of these effects. Double tourniquets are used for IV-required anesthesia (Bier blocks).18 Individual variations such as age, vascular supply of the limb, condition of the tissues, and vascular diseases all influence the patient’s tolerance to tourniquet usage. In general, avoid using tourniquets in trauma cases except where dissection around major nerves is required.
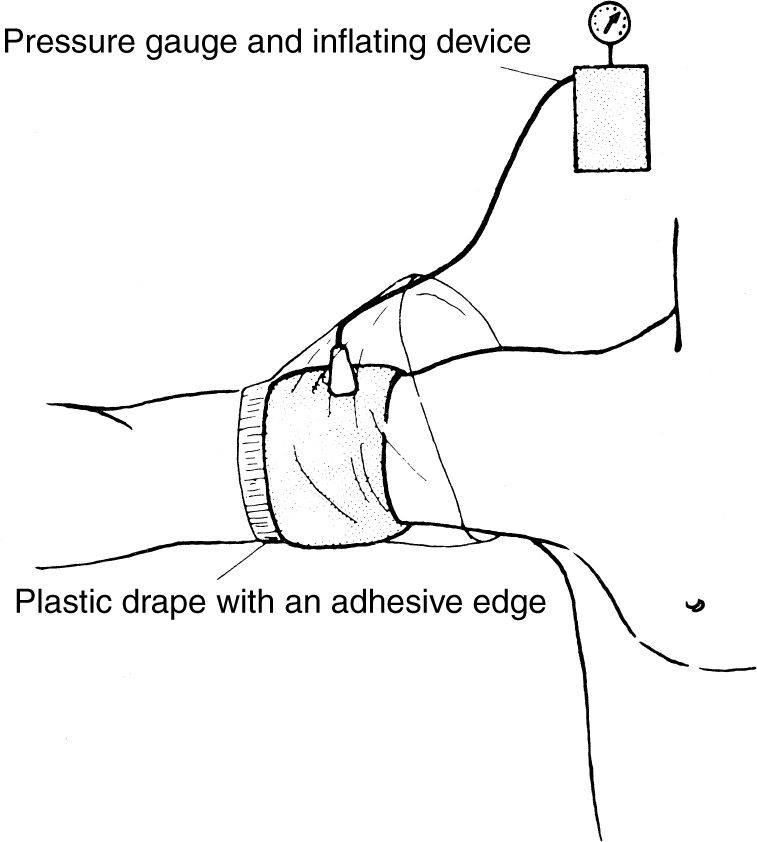
Figure 11-1. Application of a pneumatic tourniquet.
c. Complications of tourniquets include blisters and chemical burns (from “prep” solutions that leak under the tourniquet) of the skin, swelling, stiffness, and paralysis. Electromyographic changes have been demonstrated following the use of a tourniquet even within the approved time ranges.
3. The following is a summary of Occupational Safety and Health Administration (OSHA) regulation No. 1920, “Bloodborne Pathogens,” emphasizing staff and surgeon responsibilities.
a. Wash hands immediately after removing gloves.
b. Wash (with soap and water) any exposed skin (or flush mucous membranes) immediately (or as soon as feasible) after contact with blood or potentially infectious materials.
c. Do not bend, cut, recap, or remove needles or other sharps. If recapping is the only feasible method, it must be done using a mechanical device or the one-handed method.
d. Do not eat, drink, smoke, apply cosmetics or lip balm, or handle contact lenses in work areas where there is a reasonable likelihood of occupational exposure.
e. Perform all procedures involving blood or potentially infectious material to minimize spraying and splattering.
f. If outside contamination of transport containers is possible (or there is a potential for puncture), place potentially infectious material in a second container to prevent leakage during handling.
g. Use personal protective equipment such as gloves, face shields, masks, gowns, shoe covers, and so on in situations in which there is risk of exposure to blood or potentially infectious material.
h. Following an exposure, complete an incident report identifying the route of exposure and source individual. A tube of the patient’s blood should be drawn, labeled “spin” and held until the patient’s consent can be obtained. The employee health nurse is to be contacted for testing as indicated.
i. Hepatitis B virus immunization is recommended for all employees and is usually available by contacting the employee health nurse. The authors believe that every surgeon is responsible for knowing his or her own human immunodeficiency virus, hepatitis B, and hepatitis C serologic status.
4. Prevention of surgical wound infections19
a. Operating room rituals are designed to decrease infection. Despite the best designs, wound contamination and subsequent wound infection continue. It is generally conceded that most wounds become contaminated; however, usually only those with devitalized tissue, large dead spaced with accumulating hematoma, or foreign bodies become frankly infected. A study of the possible sources of coagulase-positive staphylococci that contaminated surgical wounds during 50 operations revealed that bacteria of bacteriophage types that were present only in the air were found in 68% of the wounds; 50% of wounds contained bacteria of bacteriophage types that were found in the patient’s nose, throat, or skin; 14% had bacteriophage types found in the noses and throats of members of the scrubbed surgical team; and 6% of the wounds had bacteriophage types found on the hands of the scrubbed surgical team. Maximum contamination occurs early in the operative procedure when there is a considerable amount of air circulation caused by individuals moving about the room.3 After the air quiets, the rate of contamination is less, but an increased exposure time allows increased contamination. It is important to keep traffic in the operating room to an absolute minimum, to walk slowly, and to avoid fanning the air with quick opening of the doors, drapes, and towels.
b. Studies show considerable variation in the filtration efficiency of different masks. Cloth masks are only about 50% efficient in filtering bacterial organisms and are rarely used. Numerous disposable masks have a bacterial filtration efficiency greater than 94% according to the manufacturers. Fiberglass-free masks are probably safer. Prolonged use (averaging 4½ hours of operation time) and the use of moist masks do not impair the ability to filter, except in the case of cloth masks. As the surgical masks work on a filtration principle, double masking can actually increase the air contamination with bacteria because double masking makes transportation of air through the mask pores more difficult and forces more unfiltered air to escape along the sides of the mask.
c. Although airborne contamination is by far the most important source of contamination, skin contamination does occur. Even with the use of 1% or 2% tincture of iodine, the deeper areas of the epidermis are not bacteria free. With a 1% concentration, no cases of skin irritation have occurred. If a higher concentration is used, however, the excess iodine should be removed with alcohol after 30 seconds. One 5-minute scrub with povidone-iodine is as effective as a 10-minute scrub in reducing bacterial counts on the skin and keeping them down for as long as 8 hours. A 7.5% povidone-iodine (Betadine) skin disinfectant yields 0.75% available iodine. More recent work shows that chlorhexidine gluconate (Hibiclens) may be the scrub detergent of choice for both the surgeon and the patient.20–22 A comparative study among hexachlorophene (pHisoHex), povidone-iodine, and chlorhexidine showed the latter to be probably the most effective. There was a 99.9% reduction in resident bacterial flora after a single 6-minute chlorhexidine scrub. The reduction of flora on surgically gloved hands was maintained over the 6-hour test period. In addition, the pharmacology of chlorhexidine is reportedly more effective against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa.
d. Extremity draping. Adhesive plastic drapes do not totally eliminate the patient’s skin as a possible source of infection. Drape the extremities as described in Appendix E.
e. Intraoperative procedures to prevent postoperative wound infection include the elimination of any large collection of blood. A hematoma is an excellent potential culture medium. Wound suction is used whenever one anticipates continued bleeding into the wound; however, their use in fracture, joint replacement, and spine surgery has not been proven to decrease the incidence of wound infection. Surgical wounds are carefully irrigated to remove any potential contaminated residue before closing. In vitro experiments using bacitracin 50,000 units plus polymyxin B sulfate (Aerosporin) 50 mg in a liter of saline or lactated Ringer solution have shown that 100% of Staphylococcus aureus, Escherichia coli, the Klebsiella organisms, and P. aeruginosa bacteria were killed by a 1-minute exposure to the antibiotic solution.23 Staphylococcus epidermidis organisms were also killed. Only the Proteus organisms showed significant resistance to this antibiotic irrigation (only 3% to 22% were killed). Proteus organisms are uncommon as a cause of immediate postoperative infections in musculoskeletal surgery, however, when the wounds are not previously contaminated or infected. Data indicate that irrigation of surgical wounds with a solution containing bacitracin and polymyxin B sulfate or bacitracin and neomycin could potentially lower the incidence of postoperative infections.24 A large number of patients are sensitive to neomycin, so its use is generally discouraged. Polymyxin B is sometimes difficult to obtain from the manufacturer. In this situation, some surgeons use a dilute Betadine solution as a topical antibiotic irrigant; however, this solution is toxic to tissue. Data confirming that antibiotic irrigants are superior to sterile saline in preventing surgical wound infection are generally lacking in orthopaedic surgery. Splash basins are a source of bacterial contamination and should not be used.
f. The incidence of infection increases in wounds open for longer than 2 hours. Whether this is a result of the increased exposure to the air, failure of masks, skin contaminants, or more trauma in the wound is not certain. Even with lengthy surgical cases, with good surgical technique the rate of deep wound infection on “clean” orthopaedic cases should not exceed 1%.
g. Laminar air flow systems appear to be an effective means of reducing postoperative infection rates as long as the flow of air is kept laminar or streamlined across the operative area (e.g., during hip surgeries). These systems are not effective if the air becomes turbulent across the operative area because, for example, of the position of people in the operating room (e.g., during knee replacement surgery).25
h. Hooded surgical exhaust systems are effective but can be cumbersome.
i. Whenever a subsequent surgical wound infection occurs in a clean, uneventful surgical case (particularly 2 to 3 cases within a month or two), consider a nasal culture from all those present at the time of the procedures.
5. Malignant hyperthermia
a. Pathophysiology. The target organ in malignant hyperthermia is skeletal muscle. Certain triggering events, such as the administration of volatile anesthetics or succinylcholine, precipitate release of calcium from the calcium-storing membrane (sarcoplasmic reticulum) of the muscle cell. The abnormal transport of calcium results in recurrent sarcomeric contractions and consequent muscle rigidity. The metabolic rate is accelerated, causing heat and increased carbon dioxide production with accelerated oxygen consumption. Core body temperature increases.
b. History. The potentially fatal syndrome is an autosomal dominant metabolic disease. In 40% of reported cases, an orthopaedist is the first to encounter this disorder. The incidence in the United States is approximately 1:1,000. The syndrome is associated more frequently with patients having congenital and musculoskeletal abnormalities: kyphosis, scoliosis, hernia, recurrent joint dislocations, club foot, ptosis, or strabismus. Malignant hyperthermia can occur at any age but is most likely to occur in a young individual. After exposure to an anesthetic (or other stress), body temperature may rapidly increase.
c. Examination. A rapid elevation in body temperature is noted early; however, it may become present late or not at all. Cardiac arrhythmias are usually concurrent, can progress to ventricular tachycardia, and may end in ventricular fibrillation with subsequent death. The soda lime canister may turn blue and become palpably hot. Tetanic muscle contractions occur in approximately 60% of cases. Like so many conditions in orthopaedics, early recognition is crucial. Temperature and electrocardiographic monitoring during surgery is mandatory. A rapid temperature elevation (even from an initial subnormal temperature), tachycardia, hypertonia of skeletal muscle, unexplained hyperventilation, overheated soda lime canister, dark blood, sweating, and blotchy cyanosis are all indicative of possible malignant hyperthermia.
d. Treatment
- Prevention
(a) Obtain a careful past history and family history, inquiring especially about fatal or near-fatal experiences following emotional, physical, traumatic, or surgical stress or about a relative who died of an obscure cause in the perioperative period.
(b) Dantrolene (approximately 12 mg per kg body weight) used IV is one of the mainstays of treatment and probably works by reducing calcium outflow from the sarcoplasmic reticulum into the myoplasm.
(c) Avoid the use of volatile anesthetics (Fluothane) and succinylcholine (Anectine) in high-risk patients.
- Management of an evolving malignant hyperthermia syndrome
(a) Immediately discontinue all anesthetic agents and muscle relaxants and terminate the surgical procedure as quickly as possible.
(b) Hyperventilate with oxygen.
(c) Use IV sodium bicarbonate, 4 mL per kg body weight, and repeat as necessary until blood gases approach normal.
(d) Administer mannitol, 1 g per kg body weight and furosemide (Lasix), 1 mg per kg body weight, which help maintain urine output to clear myoglobin and excessive sodium.
(e) Treat hyperkalemia with approximately 50 mg of IV glucose with 50 units of insulin.
(f) Control arrhythmias.
(g) Cool the patient with immersion in ice water and expose to an electric fan to facilitate evaporation. Refrigerated saline or Ringer lactate administered IV is helpful. Maintain cooling procedures until the body temperature is less than 38°C.
(h) Physiologic monitoring by electrocardiography and measurement of the central venous pressure, blood gases every 10 minutes, volume and quality of renal output, serum electrolytes, glucose, serum glutamic oxaloacetic transaminase, creatine phosphokinase, and blood urea nitrogen is important.
(i) Good prognostic signs are lightening of the coma (often heralded by restlessness), return of reflexes, return to normal temperature, reduced heart rate, improved renal output, and return of consciousness.
e. Complications
- Weakness and easy fatigability persist for several months.
- Death owing to ventricular fibrillation can occur within 1 or 2 hours from the onset of the condition. If death occurs later, it is usually a result of pulmonary edema, coagulopathy, or massive electrolyte and acid–base imbalance. If the patient dies after several days in a coma, the cause is usually renal failure or brain damage.
II. ORTHOPAEDIC OPERATING ROOM INSTRUMENTS AND THEIR USAGE
A. Introduction. Much of the remaining discussion is modified from a psychomotor skills course originally organized for the University of Washington Department of Orthopaedic Surgery residents by F. G. Lippert III, M.D., in the 1980s.
B. Techniques for checking the function of grasping type surgical instruments.26 The breakdown of high-quality instruments is often the direct result of their misuse. Forceps, hemostats, needle holders, and clamps are frequently misused in orthopaedic surgery. They can be misapplied to various pins, nails, screws, and plates when pliers are not readily available. They are also misused to clamp large sponges, tubing, and needles.
- It is annoying to a surgeon and hazardous to the patient when forceps or a hemostat springs open. This mishap is caused by forceps malalignment, worn ratchet teeth, or lack of tension at the shanks.
a. Start the equipment check by visually checking jaw alignment by closing the jaws of the forceps lightly. If the jaws overlap, they are out of alignment. Then, determine whether the teeth are meshing properly on forceps with serrated jaws. In addition, try to wiggle the instrument with the forceps open and holding one shank in each hand. If the box has considerable play or is very loose, the jaws are usually malaligned and the forceps need repair.
b. To check the ratchet teeth on instruments, clamp the forceps to the first tooth only. A resounding snap should be produced. Then hold the instrument by the box lock and tap the ratchet teeth portion of the instrument lightly against a solid object. If the instrument springs open, it is faulty and needs repair.
c. Test the tension between the shanks by closing the jaws of the forceps lightly until they barely touch. At this point, there should be clearance of 1/16 in or ⅛ in between the ratchet teeth on each shank.
2. To test the function of the needle holder,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree