Fig. 1
Dissection tools
8.
10 ml syringes.
9.
1.5 ml Eppendorf tubes.
10.
Phosphate-buffered saline (PBS): 1.54 mM KH2PO4, 155.17 mM NaCl, 2.70 mM Na2HPO4, pH 7.4.
11.
Regular plastic dishes.
12.
Dissecting dish (e.g., Electron Microscopy Science, Cat. No. 70540).
13.
Microscope with camera (e.g., Olympus BX41 with Olympus UC30).
14.
Fixation solution: 4 % paraformaldehyde (PFA) in PBS. For 500 ml, heat 400 ml PBS in a fume hood to approximately 60 °C on a hot plate with a magnetic stirrer, add 20 g of paraformaldehyde, and then add 1 M NaOH dropwise until all powder is dissolved. Make up to 500 ml with PBS, cool the solution, filter and adjust pH to 7.4. Use fresh or store frozen at −20 °C.
15.
Fridge (or 4 °C cold room).
16.
0.2 % ORO solution (w/v): Dissolve 0.07 g ORO in 25 ml 100 % methanol mixed with 10 ml 1 M NaOH (see Note 1 ).
17.
Glass funnel.
18.
0.45 μm filter paper.
19.
Methanol:
(a)
78 % methanol in distilled water.
(b)
100 % methanol.
20.
Roller and tilted mixer.
21.
Histosec paraffin (paraffin enriched with polymers).
22.
Oven with temperature range 1–180 °C (e.g., Memmert 100-800).
23.
Paraffin-embedding station.
24.
Histology supplies:
(a)
Tissue processing/embedding cassettes with lids.
(b)
Pencil.
(c)
Biopsy foam pads.
(d)
Metal or plastic base molds.
(e)
Superfrost microscope slides.
(f)
Glass cover slides.
(g)
Slide storage boxes.
(h)
Slide-staining racks and jars.
(i)
Fine paintbrush.
(j)
Forceps.
25.
Microtome.
26.
Stainless steel microtome blades.
27.
Histology paraffin bath with temperature range 0–100 °C (e.g., Termofin, PSelecta).
28.
Ice bucket.
29.
Xylene.
30.
Fume hood.
31.
Harris-modified hematoxylin solution (e.g., Sigma, Cat. No. HHS32).
32.
Eosin Y alcoholic solution (e.g., Shandon, Cat. No. 6766008).
33.
1 % acetic acid in distilled water.
34.
Xylene-based mounting medium: 45 % acrylic resin and 55 % xylene.
35.
Stereomicroscope fitted with a digital camera and cold light (e.g., Olympus SZX10 with Olympus UC30 and Olympus KL1500 Compact).
36.
Computer.
37.
Software for bright-field image capture (e.g., Olympus Soft Imaging Solutions).
38.
Software for image analysis and quantification (e.g., SigmaScan Pro 0.5).
3 Methods
3.1 Dissection of the Mouse Thoracic Aorta, Aortic Arch and Heart
2.
Euthanize the mouse in a CO2 chamber.
3.
Immobilize the mouse body before dissection: with the mouse facing up, pin down (e.g., with syringe needles) the fore and hind limbs to a cork board covered with 3–4 layers of paper towels.
4.
Clean and wet the mouse fur to minimize its interference: spray a small volume of 70 % ethanol over the mouse abdomen.
5.
Using blunt scissors and forceps, cut the mouse skin from the base of the abdomen to the top of the thorax.
6.
Open the abdominal wall below the ribcage.
7.
Lift the sternum with forceps and cut the diaphragm.
8.
Open the ribcage bilaterally until the thymus and lungs are exposed.
9.
Remove the esophagus and lungs to expose the heart and gain better access to the aorta.
10.
Make 2–3 small incisions in the liver for drainage.
11.
Wash out the blood from all organs using a 10 ml syringe loaded with PBS and fitted with a 25 G needle: introduce the needle into the apex of the heart left ventricle and gently depress the plunger to pump 10 ml of PBS.
12.
Gently dry the dissection area with paper towels.
13.
Using fine scissors and forceps, grip the segment of the diaphragm that is attached to the end of the thoracic aorta, and cut the connective tissue between the aorta and thoracic cavity muscle wall to expose the aortic arch area.
14.
Cut the left and right carotid arteries and the left subclavian artery to give free access to the heart and aorta (see Fig. 2a).
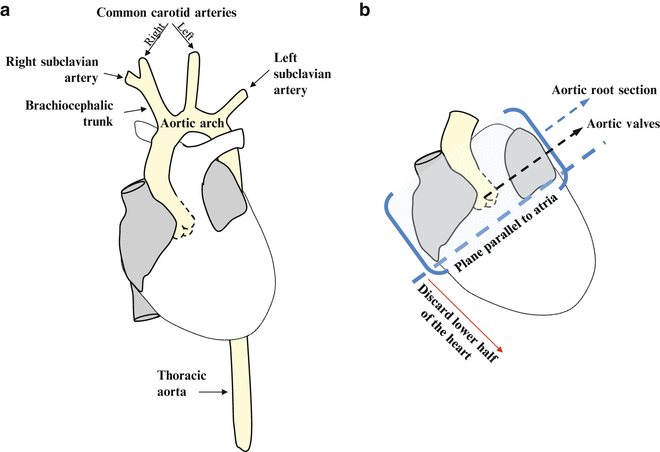

Fig. 2
Schemes for guiding aorta dissection (a) and aortic root sectioning (b)
15.
Dissect out heart and aorta and place them in a regular plastic dish containing PBS.
16.
Under a stereomicroscope, dissect the aorta by cutting at the point where it emerges from the heart.
17.
Place the aorta in a 1.5 ml Eppendorf tube, add 1 ml of freshly prepared fixation solution, and leave the tube at 4 °C overnight or up to 24 h (see Note 2 ).
18.
19.
The aorta can either be paraffin embedded (Subheading 3.4, step 3) or processed for ORO staining (Subheading 3.2). For ORO staining, you will need to clean the aorta by removing all adventitial fat under a stereomicroscope.
Place the aorta in a dissecting dish, taking care to maintain it wet at all times with PBS.
Pull away the adventitial fat very carefully with small forceps, avoiding excessive manipulation of the tissue.
Cut the branches of the brachiocephalic trunk, left carotid, and left subclavian arteries around 2 mm away from where they emerge.
20.
Place cleaned and fixed aortas in 1.5 ml Eppendorf tubes containing PBS at 4 °C and proceed with Subheading 3.2.
3.2 Staining of Aorta with ORO to Visualize Atherosclerotic Lesions
Work at room temperature.
1.
Place the cleaned and fixed aortas in 1.5 ml Eppendorf tubes, one aorta per tube.
2.
Add 1 ml of 78 % methanol to each tube and place it on a tilted roller with gentle movement for 5 min. Replace the methanol solution and repeat this step twice.
3.
Discard the methanol and add 1 ml of fresh ORO solution.








