Chapter 43 Nontransfusion Significance of ABO and ABO-Associated Polymorphisms
 Background
Background
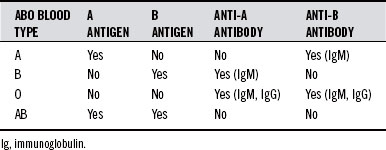
All humans can be typed for ABO blood group. There are four basic blood types: A, B, AB, and O. The system is composed of two antigens and two antibodies. The specific combination of these four components determines that individual’s type. Table 43-1 shows the possible permutations of antigens and antibodies with the corresponding ABO types.
ABH Antigens
The ABO blood group antigens are not primary gene products but instead enzymatic reaction products catalyzed by enzymes called glycosyltransferases. As depicted in Figure 43-1, they are synthesized from an oligosaccharide intermediate, “H substance”, which is produced by the monosaccharide fucose. Group A or B activity is produced by the addition of a single sugar on the nonreducing end of the H chain. Adding the glycoprotein N-acetyl galactosamine (GalNAc) to the end of the chain results in blood group A antigenicity. The terminal carbohydrate and the B group antigen is the monosaccharide galactose. There is no true O antigen: the terminal carbohydrate of O (H) antigen is the monosaccharide fucose. We identify the blood types as “ABO” but typically refer to the actual antigen system as “ABH.”
ABH substances manifest quite early in life processes.1 They are detected in the cell membranes and secretions of human embryos at about 5 weeks’ gestational age in the epithelium and virtually all vascular endothelium. In fetal organs’ endothelial cells, the expression of ABH antigen is ubiquitously upregulated. This is considered suggestive evidence that blood group antigens serve as early immunomorphologic markers of the endothelial differentiation of mesenchymal cells, specifying the location of future blood vessels.2
The embryonic expression of ABH antigens may be an important aspect in the pathogenesis of certain diseases: a scrutiny of 2557 medical records for children with type 1 diabetes and controls showed ABO blood group incompatibility in close to 90% of children with diabetes,3 indicating that maternal–fetal ABO incompatibility may have acted to inhibit normal pancreatic islet formation in the fetus.
The expression of ABH antigens in the adult is tightly regulated, and their reappearance in adult tissue—normally devoid of them—is virtually always a sign of disease. Inappropriate ABH expression is one of the prime manifestations of the aberrant glycosylation state that is a hallmark of malignancy. Instances have been observed indicating that aberrant ABH expression may be paralleled in entirely different organ systems. A link has been demonstrated between ABH antigen expression in normal and neoplastic colonic epithelia and consequent alterations of ABH expression in the thyroid.4
ABH Secretors and Nonsecretors
ABH substances are secreted by mucous glands in many organs, including the upper respiratory tract, the gastrointestinal tract from the esophagus through the colon, and the uterine cervix. ABH secretor status is a major conditioner of the gut mucosa. ABH secretors have greater quantities of free ABH antigens in the makeup of their intestinal secretions, which has significant effects on bacterial and lectin adherence to the gut microvilli. The secretor gene regulates the synthesis of blood group substances in superficial glands of the gastric and small intestine mucosa. Large amounts of ABH material are found in all secretors,5–8 characterized by a uniform distribution of blood type antigens in the gastric pits. ABH expression is independent of secretor status: glands situated deep in the mucosa of the pylorus and small intestine (Brunner glands) and gastric parietal glands both produce A and B substances without regard to secretor status.9
For a more detailed discussion of the metabolic consequences of ABH secretor status, the reader should refer to the author’s article specifically on the subject.10
Brush-Border Hydrolases
ABO blood group determines much of the enzyme activity in the tissue (brush border) of the intestine. At least six intestinal hydrolases have ABO blood group antigenic determinants directly related to ABO blood group. The expression of ABH antigens secreted by intestinal glycoproteins is under the control of the secretor gene; therefore, these antigens are not detected in the hydrolases of nonsecretor subjects.11
Intestinal Alkaline Phosphatase Activity
Intestinal alkaline phosphatase (IAP) is involved with both the breakdown of dietary cholesterol and the absorption of calcium. The activity of IAP and serum alkaline phosphatase (SAP) is strongly correlated with ABH secretor phenotypes. It has been estimated that the SAP activity of nonsecretors is only about 20% of the activity in the secretor groups.11–15
ABO polymorphism is linked to the levels and persistence of IAP.16 Numerous studies have associated group O individuals with the highest alkaline phosphatase activity, and group A with the lowest.17 In addition, one study implied that the group A antigen itself might inactivate IAP.18
Bacterial Flora
The role of the ABO blood group in determining the bacteria making up a healthy gastrointestinal ecosystem is particularly strong in ABH secretors. Because ABH secretor status and ABO blood group dictate the presence and specificity of A, B, and H blood group antigens in human gut mucin glycoproteins, their status can influence the populations of bacteria capable of taking up local residence. This occurs because some of the bacteria in the digestive tract are actually capable of producing enzymes that allow them to degrade the terminal sugar of the ABH blood type antigens for a constant food supply.19 Bacteria capable of degrading blood group B antigen can detach the terminal α-D-galactose. Group A–degrading bacteria can detach the GalNAc. These bacteria have a competitive advantage and can thrive in the environment created by the preconditioning of ABH secretions. Although comparatively small populations of bacteria produce blood group–degrading enzymes (estimated populations are 108 bacteria per gram), the quantity of these bacteria are several orders of magnitude greater in different blood types, and they are much more stable residents. For example, B-degrading bacteria have a population density about 50,000-fold greater in blood group B secretors than in other subjects. Similar bacterial specificity and enzyme activity are found in other blood types.20
Evidence suggests that ABH nonsecretors have lower levels of immunoglobulin-G (Ig-G)21,22 and secretory IgA concentrations than secretors.23,24 ABH nonsecretors appear to have a higher prevalence of a variety of autoimmune diseases, including ankylosing spondylitis, reactive arthritis, psoriatic arthropathy, Sjögren’s syndrome, multiple sclerosis, and Graves’ disease.22,25–27
Blood Group as Self-Declaration and Adhesion Molecules
Glycans can have a significant effect on fungal, viral, and bacterial pathogenicity.28 ABH antigens are ubiquitous in nature, found abundantly in foodstuffs (where they are thought to play a role in the induction of opposing blood group antibodies early in life) and in a host of microorganisms.
A 1995 study showed that of 833 fungi harvested from 1977 to 1994, 422 extracts (47.8%) produced agglutination of human red blood cells (RBCs), equally distributed against type O, A, and B cells. The fungal agglutinins, in this case, are desirous of attaching and infecting seeds or other microbes that possess some “ABO blood type” activity of their own.29
ABH antigens appear in secretions by 8 to 9 weeks of age, first in the salivary glands and stomach, then throughout the gastrointestinal and vaginal tracts. The ABH variation of blood group antigen expression on vaginal epithelial cells and mucus has a significant role in susceptibility to urinary tract infections in women.30
Infertility and Spontaneous Abortion
ABO blood type incompatibility may be a critical factor in infertility. ABO-incompatible mating couples (a type A male fertilizing a type O female) are a common occurrence in miscarriages, especially very early in the gestational term. One study of 288 miscarriages showed that there was an excess of blood type A and type B in otherwise normal fetuses. The researchers concluded that the ABO incompatibility between mother and fetus was likely a cause of early miscarriages, but almost exclusively in chromosomally normal fetuses.31–34
In a study of 102 infertile couples, Solish35 found that 87% were blood type incompatible. This researcher suggested that the infertility was due to the presence of antibodies in the secretions of the mother’s genital tract or incompatible sperm from the father. In another study, a total of 589 compatible mating couples were compared with 432 incompatible mating couples. The mean number of living children presented a significant difference. There was a 21% deficiency of type A children in the two groups. Similarly, there was a 16% deficiency of type B children in the two groups. It appears that a 31.9% rate of miscarriage is associated with incompatible matings, compared with 17.15% in compatible matings. This finding has led some researchers to theorize that ABO incompatibility results in “cervical hostility” between the man’s blood type antigens, which are present in his sperm, and the woman’s opposing antibodies, present in her cervical mucus.36
Besides infertility, habitual abortion is also related to ABH secretor status as shown in 2010. Secretor status of 66 couples with recurrent spontaneous abortion (RSA) was obtained and compared with couples with successful term pregnancies. It was found that a secretor phenotype of couples with RSA, especially of the husband, could facilitate “reproductive success” and that couples in which both parents were secretors, the RSA probability was extremely diminished.37 A previous study showed the interaction between ABO blood groups and adenosine deaminase genetic polymorphism during intrauterine life, providing results that could not be explained by strictly conventional immunologic mechanisms. The authors concluded that cell-to-cell interactions involving ABO antigens might have an important role at implantation, and that adenosine deaminase, through control of local adenosine concentration, could modulate these interactions, influencing the probability of successful implantation.38 A third and most recent study comparing secretor status compatibility between mother and child suggested an intrauterine selection against Se- (nonsecretor) of the embryo carried by an Se+ (secretor) mother. The selection was dependent on factors influencing the maternal environment.39
To measure women’s fertility below age 45, follicle-stimulating hormone (FSH) levels are measured. Women with an FSH level greater than 10 are considered to have diminished ovarian reserve. In an ongoing study at Yale University and the Albert Einstein College of Medicine, it was observed that women with types A or AB blood were significantly less likely to have an FSH greater than 10 than were women with types O or B blood. They concluded that type O blood might affect fertility.40
Other Immune Correlates
In 1991, D’Adamo reported that individuals of group O blood reporting previous urticaria or anaphylaxis showed high residual titers of anti-A isoagglutinins. A score value was assigned to each agglutination reaction. Individuals with these disorders showed remarkably high titration scores compared with controls of the same blood group, up to three times higher. Mild increase was noted for group O subjects with severe eczema or asthma, but total scores in these subjects were only marginally greater than those of controls. A striking association was shown for group O women experiencing endometriosis.41
ABO and Secretor Blood Group Genetics
ABO System
In 1910 Epstein and Ottenberg suggested that the ABO blood group system could be inherited.42 The determination of ABO status is the result of two co-dominant alleles and one recessive allele found on chromosome 9q band 34.
ABO genes consist of at least seven exons, and the coding sequence in the seven coding exons spans more than 18 kb of the genomic DNA. The single nucleotide deletion found in most (but not all) of the O alleles that is responsible for the loss of the activity of the enzyme is located in exon 6.43
Lewis Blood Groups and Their Association with the ABH Secretor System
In Lewis-positive phenotypes, Lea is formed initially, and in the case of nonsecretors (lacking the Se gene, FUT2), Lea glycan is attached to the red cell, and they type as Lea. In the case of secretors, the Se gene activates the H gene, which causes an additional sugar to be added to Lea, converting it to Leb (see Figure 43-1).
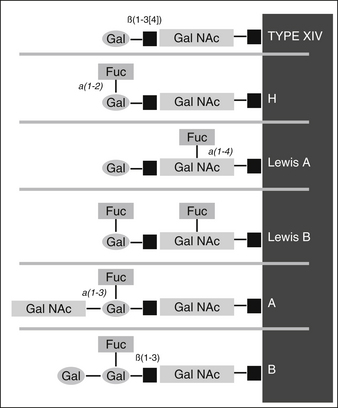
FIGURE 43-1 Structures of the nonreducing end of the ABH and Lewis blood group antigen determinants.
Thus, it is often possible (and quite handy) to use the Lewis groups to infer ABH secretor status, because Lewis typing is fairly quick to perform and easy to master compared with the traditionally used salivary inhibition test. However, using Lewis typing to infer ABH secretor status works only in those individuals who are Lewis-positive (about 9 of every 10 patients). Lewis-negative individuals can either be secretors or nonsecretors. Lewis-negative patients carry important metabolic consequences of their own, worthy of much attention.10 Table 43-2 shows Lewis blood types and their relationship to ABH secretor and/or nonsecretor status.
TABLE 43-2 Lewis (Le) Blood Types and their Relationship to ABH Secretor and/or Nonsecretor Status
| LEWIS TYPE | CATEGORY | ABH SECRETOR STATUS |
|---|---|---|
| Lea+b− Lea antigen but not Leb | Lewis positive | ABH nonsecretor |
| Lea−b+ Leb antigen but not Lea | Lewis positive | ABH secretor |
| Lea−b− Neither Lea nor Leb | Lewis negative | Lewis outcome cannot determine ABH secretor status |
Individuals of blood group O phenotype run an approximate 1.5- to 2-fold higher risk for development of peptic ulcer disease,44 although there is no direct correlation between ABO blood group phenotypes and the prevalence of Helicobacter pylori infection. However, in addition to H type 1, the Leb antigen is also a binding receptor for H. pylori, and in this capacity, it can best be described as a “virulence promoting factor.” For virulent strains, Leb antigen binding activity targets the microbes to the epithelial cell surfaces and potentiates the effect of secretion of virulence factors, such as the vacuolating cytotoxin and/or neutrophil activating–recruiting factors.45
Linkage and Pleiotropism
Linked genes occur on the same chromosome and are inherited as a single unit. Gene linkage analysis shows several genes linked to the ABO locus. For example, there are strong indications that a gene regulating dopamine β-hydroxylase activity is linked to the ABO blood group locus.46 Dopamine β-hydroxylase is a key enzyme in the conversion of dopamine to norepinephrine.
This linkage may help explain the continued significance of ABO group as a discreet and significant genetic marker for a variety of affective disorders, including type A behavior in men subsequent to myocardial infarction47 and bipolar depression,48,49 each of which has been associated with blood group O. The ABO locus shows putative linkage with platelet monoamine oxidase activity,50 reduced levels of which have been noted in group O healthy men.51
Additional evidence implies that there is a linkage between the ABO gene and the gene that regulates the activity of the enzyme argininosuccinate synthetase, which recycles arginine from citrulline in the production of nitric oxide.52 A letter to the editor in the journal Lancet reported differences between ABO groups in their responsiveness to inhaled nitric oxide therapy: types with a B antigen (B and AB) had less success with this therapy.53
Elevated factor VIII (FVIII) levels contribute to venous thrombotic risk. FVIII levels are determined to a large extent by levels of von Willebrand factor (vWF), a protein that protects FVIII against proteolysis.54 ABO polymorphism is one of the best-characterized genetic modifiers of plasma FVIII; it accounts for approximately 30% of the total genetic effect.55 Subjects with blood group non-O have higher vWF and FVIII levels than individuals with blood group O.56
Digit ratio, a marker of assessing the levels of prenatal exposure to androgens, has yielded mixed results57,58 as a predictor of androgen stimulation in individuals with polycystic ovarian syndrome. However, preliminary evidence suggests that genes contributing to the expression of 2D:4D reside in the vicinity of the gene loci (chromosomal locations: 9q34.2 and 1p36.11) of the blood groups or that there may be pleiotropic effects on digit ratio as a result of the blood group genes. Associations using digit ratios may require incorporation of ABO blood group in their interpretation.59
Additional Physiologic Correlations
Gastric Acidity
Because the prevalence of both pernicious anemia and gastric cancer is higher in individuals of blood group A and that of duodenal ulcer higher in those of group O, a hypothesis relating blood group effects on acid secretion was inevitable.60 Early work confirmed that acid output tended to be greater in group O than in group A subjects.61,62
Gastrin and Pepsinogen
In one study, serum pepsinogen A (pepsinogen I) levels were studied in relation to ABO blood group, age, and sex in 700 healthy blood donors. Serum pepsinogen A levels were higher in males than in females and rose with increasing age. Blood group O individuals showed higher serum pepsinogen A levels than blood group A individuals.63 There is also evidence that the type A antigen in gastric juice binds to pepsin and possibly inactivates it.64 A study using serum pepsinogen levels as a marker for gastric atrophy showed a high association with blood groups A and B.65 However, possibly owing to the polygenic nature of pepsinogen activity, one study failed to find any significant difference in pepsinogen levels between ABO groups.66
Another study looking at ABO polymorphism and serum gastrin concentration after stimulation by a glycine drink could find no correlation with ABO blood group.67 However, the study had a simple preprandial and postprandial methodology. In a separate study, the concentrations of gastrin were measured in the blood of 121 fasting healthy Greek volunteers of both sexes and different ABO blood types, between ages 20 and 70 years. The testing took place immediately after a test meal was eaten by subjects who had fasted for 8 hours; the measurement was repeated at 10 and 40 minutes post meal. The researchers found that gastrin levels took 40 minutes to increase after the meal in the blood type A and B subjects, but that a significant increase appeared 10 minutes after the meal in the blood type O subjects.68
Cholesterol
Although several studies on highly select populations have yielded conflicting results,69,70 the general consensus is that blood group A individuals have a significantly higher basal cholesterol level than those in other blood groups. The relationship between ABO blood phenotype and total serum cholesterol level was examined in a specific Japanese population. The results showed that cholesterol levels were very significantly elevated in the blood type A compared with non-A.71
ABO blood group and coronary risk factor levels were measured in a nationwide sample of more than 6000 black and white adolescents aged 12 to 17 years. Blood group A was associated with significantly higher serum total cholesterol levels in white females independent of all other risk factors, in white males independent of age and weight, and in southern black females independent of age and weight.72 A separate study (the Bogalusa Heart Study) looked at 656 white and 371 black adolescents and found the same results with regard to total cholesterol; even higher levels of low-density lipoprotein cholesterol were found in type A adolescents than in other blood types.73
Stress
Several studies have identified differences between ABO group and possible chemical responses to stress. Interestingly, individuals of blood group A appeared to have a lower incidence of “type A personality.”74
One study evaluated the influence of blood type A versus O coupled with a mirror-drawing stressor on very-low-density lipoprotein toxicity-preventing activity (TxPA). Plasma cortisol levels showed significant ABO variation. Exposure to the stressor significantly decreased TxPA and increased cortisol for the total group of 25 older men. The stress response patterns of the 15 blood type A men were different from those of the 10 type O subjects. The blood type A group had higher initial levels of TxPA and cortisol as well as quicker stress recovery rates than the type O group.75 Another study showed that blood group A individuals responded to a stressful situation (venipuncture) with higher levels of cortisol and, possibly, of adrenaline.76
Rheology
There is evidence that the rheology of blood may play a role in a variety of chronic anxiety states. Compared with normal subjects, chronic depressive and schizoid patients had very significant differences in their blood rheology and in the ability of their RBCs to aggregate. When patients with schizoid anxiety were compared to those with depressive anxiety, their ratio of albumin to globulin was increased. When patients were divided according to their ABO blood groups, significant differences were found in their albumin/fibrinogen ratio and their blood viscosity. This was particularly true for women who had blood type A and who had depressive anxiety; their blood tended to be substantially “thicker” and to have higher amounts of serum proteins in it than women with similar depression who had blood type O.77
Associations between the ABO phenotype and variations in blood rheology have also been reported in high blood pressure78; stress79; diabetes80; heart attack, cancer, and thyroid disease81; kidney failure82; and malignant melanoma.83
 Pathologic Process and ABO Blood Groups
Pathologic Process and ABO Blood Groups
Mental Disease
There are several reports exploring the relationship of ABO groups, ABH and Lewis secretor type, and mental disease like obsessional illness,85 bipolar disorder,86,87 depression,88 and schizophrenia.87 A significant part of that research was done in the 1970s and 1980s and does not seem to have been updated or explored much further. Most of the articles reviewed and not discussed here were not conclusive about blood group associations.
Cardiovascular Disease
Stroke
A European study compared 50 patients with stroke. The standard expected frequency of ABO blood types in the surrounding population showed that the frequency of the blood group A in the patients with stroke was 120% greater than would normally be expected. The percentage of blood type B was even higher (159% of expected rate of occurrence). Patients with blood type O were only 85% as likely to experience stroke.89
A 1979 study of 220 patients with stroke looked at the viscosity of their blood a few hours after the stroke event. About 80% of the patients had blood cells that easily aggregated. What was especially interesting was the discovery by the researchers that the clotting of blood in patients with A and B blood types was mostly due to fibrinogen, whereas in blood types O and AB it was caused by other clotting factors.90
Individuals with blood types A and AB have a generalized tendency toward problems associated with blood clotting, whereas problems in those with blood types B and O appear to be linked to excessive bleeding and poor clotting. This observation was verified in several studies, the largest being performed in 1460 patients with “stroke” and reported in the Lancet. In 329 cases, the cause of death was certified as cerebral thrombosis (brain clot). In the thrombosis cases, there was an excess of patients of blood types A and AB and a deficiency of those with blood types O and B. In the 482 “strokes” that were due to cranial bleeding, the reverse was true: there was a significantly higher proportion of patients with blood types O and B than of those with blood types A and AB.91
A 2009 United Kingdom systematic review tried to assess whether the effects of non-O status on thrombosis risk were of the magnitude predicted by its effect on vWF/FVIII levels. They confirmed the historical impression of linkage between some vascular disorders and non-O blood group status. They concluded that the odds ratios were similar to those predicted by the effect of ABO(H) on vWF levels on thrombosis. They proposed further work to understand and refine the effect of reducing O(H) antigen expression and therefore a more widespread adoption of ABO(H) typing.92
Peripheral Artery Disease and Venous Thromboembolism
Peripheral artery disease (PAD) occurs in approximately 12% of the adult population. The prevalence of PAD rises with age: almost 20% of people older than 70 years have the disease. E-selectin and thrombomodulin levels are always elevated in intermittent claudication, a disorder almost always found with PAD and carrying a distinct association with blood type A.93
In 125 patients experiencing venous thrombosis in a Brazilian population, a higher than expected proportion of blood group A and a lower than expected proportion of blood group O were observed among the patients.94 This is consistent with the previously discussed influence of ABO on a third soluble endothelial product, wWF, and its role in thromboembolism. Another study in 2007 confirmed than non-O blood type was independently associated with risk of venous thromboembolism, and added to the risk associated with FV Leiden.95 Even in pregnancy, a nested case–control study within a cohort of 71,729 women who gave birth to 126,783 children in Denmark showed that blood groups A and AB might be associated with increased risk estimates for venous thromboembolism in pregnancy and the puerperium.96
Heart Disease
In 1962, the Framingham Heart Study typed the blood of the surviving 4125 members of the original study group of 5209 people first examined in 1948 to 1951. The most striking observance was the lower rates of nonfatal heart disease in men aged 39 to 72 years with blood type O than in those with blood type A.97 A 1994 Polish study on patients who underwent coronary bypass surgery with highly advanced arteriosclerosis of the coronary arteries found a significantly higher number of cases with group AB and a lower number of those with group O.98 A 1981 German study of 13,175 patients showed a prevalence of blood type A in all types of heart disease examined.99
In a study of 191 coronary artery bypass candidates, investigators paradoxically found an excess of type O over type A subjects. After examining the data more closely, they concluded that the tendency of type A subjects for more ready development of blood clots (“thrombotic proneness”) led to a poorer prognosis. In essence, the blood type A subjects were missing from the study because they had already died in greater numbers, leaving a disproportionate number of type O subjects among the long-term survivors.100 In a study of male survivors of heart disease, researchers found that there were fewer patients who were type A and younger than 55 years than would have been otherwise expected.101
A 1975 Italian study of 746 patients with high blood pressure, 3258 with congenital heart disease, and 4503 with a history of heart attack found a significant lack of patients with type O blood, and a significant excess of blood type A patients in the group with myocardial infarction. The study also showed an excess of blood type A patients with high blood pressure, and a lack of patients who were blood type B.102 A 1983 study of 255 women originally investigating the effects of smoking on the rates of heart attack also found several other factors significantly associated with heart attacks in this group, including hypertension, angina pectoris, family history, diabetes mellitus, and blood type A.103
Platt et al104 examined blood type and heart attacks in two different age groups. The patients were divided into two groups: 65 years or older and younger than 65 years. The predominance of blood type A in patients with cardiac infarction was “highly significant” in both age groups (P <0.005). This study was unique in that it excluded other risk factors, such as smoking, high blood pressure, diabetes, and high cholesterol levels. When the researchers looked specifically at the older group, the predominance of blood group A in those with cardiac infarction was even higher (P<0.001). The researchers concluded, “Our investigation strongly suggests the existence of a genetic factor associated with blood group A and independent of the other risk factors, which is also responsible for a greater incidence of cardiac infarction.”104
An 8-year study of 7662 British men found that blood type A was linked to the incidence of ischemic heart disease as well as higher total serum cholesterol concentrations.105
Cancer
These and other changes provide a selective advantage for tumor cells during their progression to more invasive and metastatic forms. Some of the more studied glycoprotein modifications include:106
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree