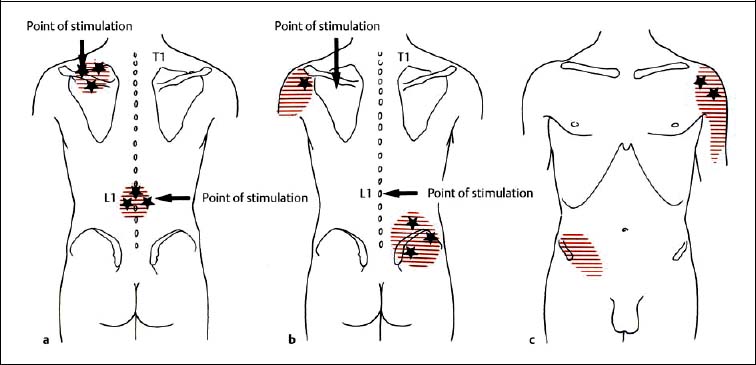
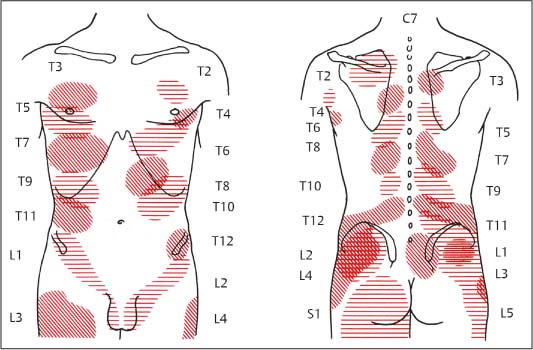
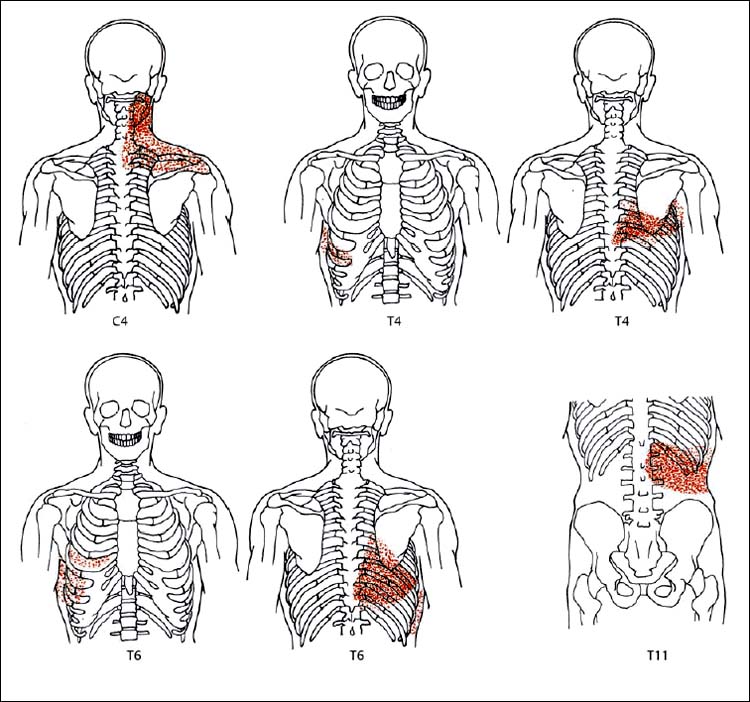
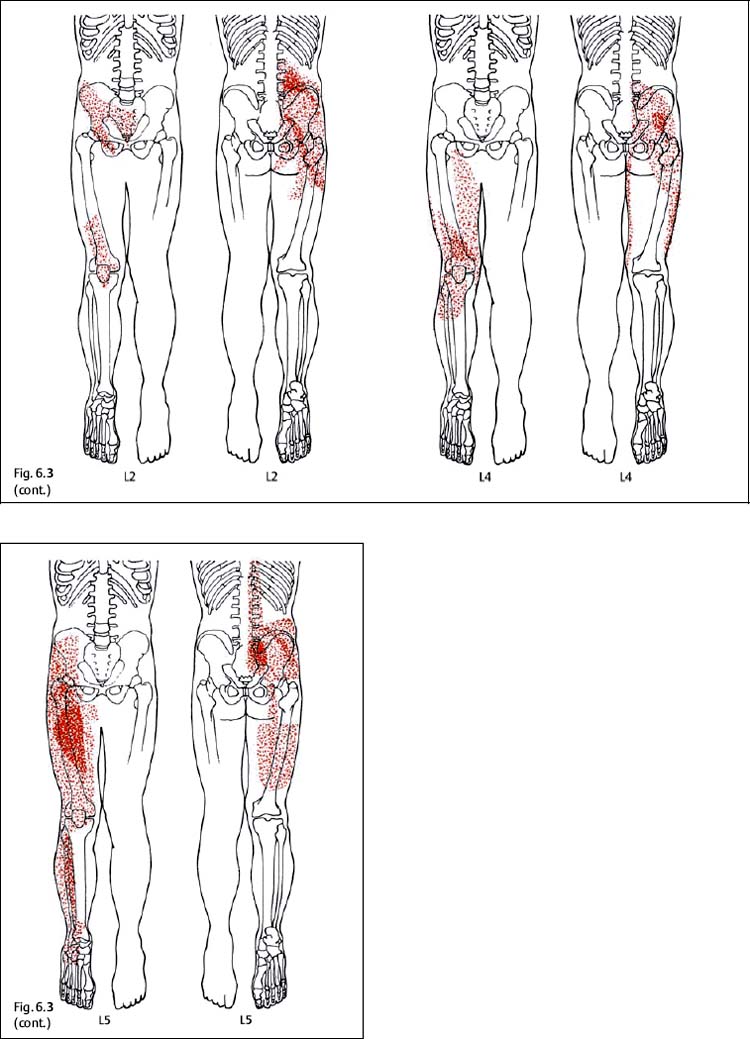
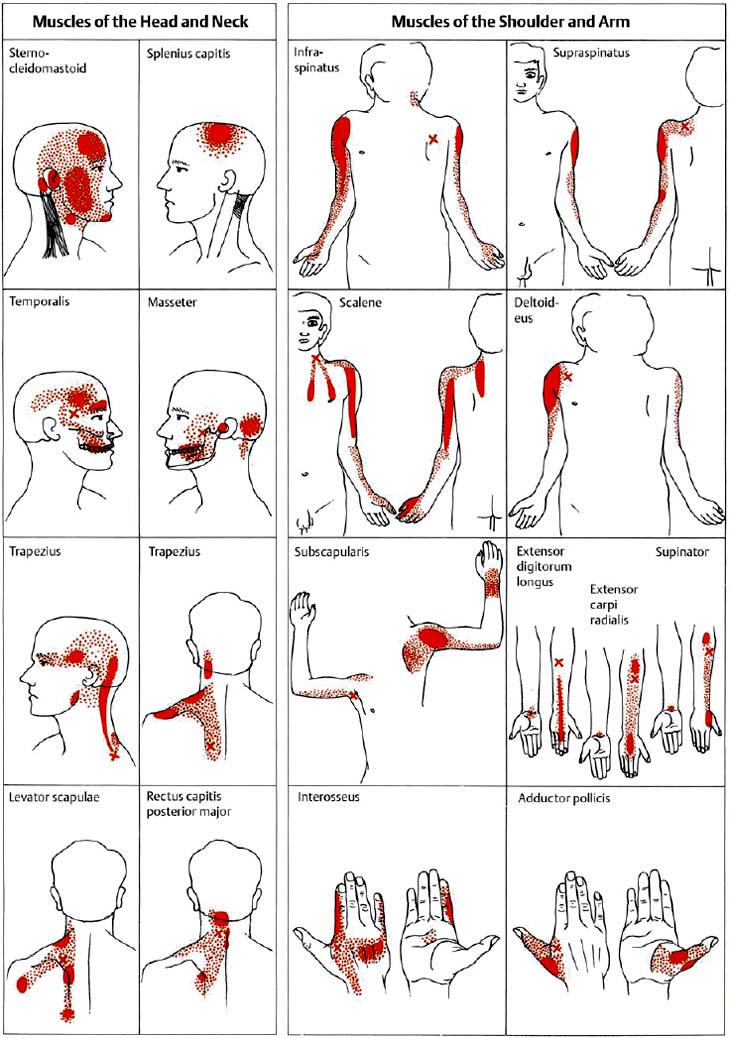
6 Nonradicular Pain: Spondylogenic and Myofascial Pain Syndromes Prior to the landmark article by Mixter and Barr (1934), who described the characteristics of sciatica following disk herniation with subsequent surgical correction, legion papers were published attempting to explain the different musculoskeletal pain syndromes, often on the basis of empirical descriptions and hypothetical postulates. Particular interest in the source of pain originating from soft-tissue structures such as muscles, ligaments, tendons, and the various articulations, in particular the sacroiliac joint, is evident in the literature up to the 1930s and 1940s. Probably all of the anatomic structures known have at one time or another been considered as the source of pain. Mixter and Barr (1934), however, rather than presenting a single case study or a listing of empirical observations, introduced a well thought-out series from the “base of the skull down, covering the ‘waterfront.’ Cervical, dorsal and lumbar lesions were all included in this first article” (Barr, 1977). Attention and effort thereafter, so it seems, were primarily directed toward the subject of the herniated intervertebral disk and its role in the radicular pain syndromes. This ultimately steered attention away from muscle and other soft tissues and toward nerve root compression as a cause of pain (Gerwin, 2000). In the most recent two or three decades, however, there has been a noticeable trend in which both the “old” or orthodox branches of medicine and the “new” or alternative health-care counterparts alike have shown particular interest in investigating many of the nonradicular pain syndromes. The exact reasons for this rising interest are not entirely clear. Many published reports have attempted to organize and categorize the seemingly countless descriptions of the apparently diverse painful soft-tissue disorders and nonradicular pain syndromes. The terminology seemed to get in the way, as many previous accounts had been categorically lumped under such nonspecific terms as inflammatory soft-tissue rheumatism. Although seemingly similar somatic phenomena appear to have been described by the various authors over the years, the language and terminology used often seemed to reflect more the individual author’s opinion or that of a particular school of thought than a common scientific language. The sheer number of the many neurophysiologic hypothetical postulates, coupled with the often confusing, nonstandard language, which also varied from country to country may, at least in part, explain why even clinically relevant observations might have been lost or hitherto ignored. From a historical perspective, this is understandable (Dvořák, 1982b, 1983; Gibson, 1980; Wardwell, 1980). The scholarly work and scientific language used by such pioneering investigators as Korr (1975) and Wyke and his colleagues (1967, 1979b) have fundamentally contributed to a better understanding of some of the neurophysiologic principles underlying musculoskeletal pain phenomena. Other articles have addressed the various clinical presentations in an effort to come up with a consistent correlation of the various functional disturbances that affect the spinal column or the peripheral joints (Brügger 1962, 1977; Feinstein et al., 1954; Hohermuth, 1981; Jones, 1981; Mitchell et al., 1979; Sutter, 1975; Sutter and Fröhlich, 1981; Waller, 1975; Dejung, 1985; Dvořák et al., 1987c; Southwick and White, 1983; Herron and Turner, 1985; Mattle, 1986). Simons (1976a, 1988) has presented an exhaustive summary of the many reports in the literature that relate the muscles and fascia to the various pain syndromes, while at the same time posing the question “Why should so common and serious a problem be so neglected by modern medicine?” (Simons, 1988). The ideal is to identify those clinically meaningful parameters and diagnostic criteria that would unequivocally assist in distinguishing from true radiculopathies the painful nonradicular musculoskeletal syndromes that mimic or are similar to the explicit radicular syndromes. This should, at least in theory, facilitate the proper choice of therapy for a particular disorder, clinical presentation, or syndrome. For the field of manual medicine this means that one goal is to determine which approach or technique (s) are best suited for a particular patient, ranging from such varied maneuvers as the high-velocity, low amplitude manipulations (thrusting techniques) to the “gentle” approaches of soft-tissue techniques, including the various trigger point and tender point approaches, myofascial release techniques, muscle energy or facilitatory techniques, among many others. There already exist a number of good, rational rehabilitation approaches that ultimately should allow the patient to undertake the transition to an independent home exercise program as soon as the clinical situation allows. At an international manual medicine seminar in Switzerland in 1983, now known simply as the “Fischingen Conference,” an attempt was made to collate, correlate, and unify the different terminologies used by the many schools of thought and practice (Dvořák and Dvořák, 1984). In this chapter we want to present five models that deal with the various components of the nonradicular spondylogenic or musculoskeletal pain syndromes as they relate to the field of manual medicine: 1. Referred pain. 2. Myofascial pain syndromes. 3. Postural pseudoradicular syndromes. 4. Somatic dysfunction and tender points. 5. Spondylogenic reflex syndromes. Despite the diversity in terminology, the attentive reader will readily recognize the overlap of, and the similarities among, the different descriptions of the various clinically observed phenomena and the postulates of the proposed neurophysiologic mechanisms. Referred pain, in the simplest terms, may defined as pain that is perceived by the patient at a location distant from or different from the structure that is presumed to be the pain generator. This broad definition includes pain referred from visceral structures and somatic structures either directly or via reflex pathways. Here we describe observations on referred pain originating from somatic skeletal (nonvisceral) structures. Kellgren (1938), Sinclair et al. (1948), and Hockaday and Whitty (1967) demonstrated in clinical experiments that local and referred pain can be elicited upon mechanical or chemical stimulation of the diverse spinal and paraspinal structures. When injecting hypertonic sodium chloride solution into the paravertebral muscles, the ligaments, and the apophyseal joints (also referred to as the facet or zygapophyseal joints), or when scratching the periosteum with a needle, localized and referred pain was consistently elicited that was in accordance with the segmental innervation (Kellgren 1938, 1939; Lewis and Kellgren, 1939) (Fig. 6.1). When the deep structures were stimulated, a rather diffuse pain distant from the origin of stimulation was observed. When certain areas of the thoracic spine were stimulated, pain was referred to both the anterior and posterior portions of the trunk in a patchwork pattern that is similar to skin hypersensitivity (Fig. 6.2) (Hockaday and Whitty, 1967). This is in distinct contrast to other types of thoracic pain that may follow a bandlike or girdle distribution as seen in some neuropathic pain states, such as pain associated with herpes zoster. Stimulation of various structures at the lumbosacral junction produced a deep pain both in the gluteal region and in the thigh, albeit, rarely below the knee. Skilled palpatory assessment of the muscles, ligaments, and fascia can help differentiate those tissue components that are affected as a result of the referred pain and those that remain untouched by it. It appears that local anesthetic agents applied to the secondarily affected tissues (the muscles, ligaments, fascia, etc.) can reduce the referred pain level at the referral site. However, the injection of a local anesthetic agent into the secondarily affected tissues does not alter the original or spontaneous local pain of the structure that was initially stimulated (e. g., the supraspinous or interspinous ligaments). In contrast, when a local anesthetic is applied to the area of the initially stimulated ligament, both the local and the referred pain may disappear (Kellgren, 1939). Kellgren summarized his experimental observations as follows: Fig. 6.1 Pain pattern upon injection of hypertonic sodium chloride solution into (a) the superficial and (b, c) the deep structures (vertebral arches, infraspinous fossa of the scapula). (After Kellgren, 1939.) Fig. 6.2 Diagram of the patchwork distribution of referred pain after stimulation of deep structures of the thoracic spine (vertebral arches, joints). (After Kellgren, 1939.) “The superficial fascia of the back, the spinous processes, and the supraspinous ligaments induce local pain upon stimulation, while stimulation of the superficial portions of the interspinous ligaments and the superficial muscles result in a diffuse type of pain. The deep muscles, ligaments, and periosteum of the apophyseal joints, as well as the joints themselves, can cause referred pain according to the segmental innervation when sufficiently stimulated.” Feinstein et al. (1954) also injected a hypertonic sodium solution into the paravertebral structures associated with the vertebral segments between C1 and S3, such as the deep muscles and ligaments. The manifestation of referred pain and the localization of zones with decreased sensitivity to pain did not always correspond to known radicular innervation patterns such as the zones described by Head, for instance. In Feinstein’s studies, the pain was felt mostly in the reflexly hypertonic muscles upon injection of the sodium salt solution. This was accompanied by autonomic symptoms, such as paleness, excess perspiration, decreased blood pressure, fainting, and nausea, among others. Figure 6.3 depicts the various observed patterns of referred pain according to the level of segmental stimulation at C4, T4, T6, T11, L2, L4, and L5. Fig. 6.3 Schematic representation of referred pain after injection of hypertonic sodium chloride solution into the deep somatic soft tissues. (After Feinstein et al., 1954.) The myofascial pain syndrome is a muscle pain disorder in one or more muscles or groups of muscles accompanied by local and referred pain, and is associated with decreased range of motion, weakness, and often autonomic phenomena. Patients are readily recognized by their history of characteristic muscle pain and the presence of myofascial trigger points, which are specific areas of hyperirritability in a muscle that cause local and referred pain on palpation (Meyer, 2002). Research during the past 20 to 30 years has provided valuable information for clinical practice about the painful syndromes associated with the myofascial trigger points (Melzack, 1978; Reynolds, 1981; Rubin, 1981; Simons 1975, 1976b; Travell and Rinzler, 1952; Travell 1976; Travell and Simons, 1992). The trigger points may be spontaneously painful or may be elicited upon digital pressure. In the presence of a true myofascial trigger point, the patient describes the perceived pain in an area that follows a pattern that is characteristic for the particular muscle harboring the myofascial trigger point (Fig. 6.4). Similarly to the phenomenon described as the irritation zone (described below), a specific anatomic or histologic substrate has not yet been identified nor have any particular neurophysiologic interactions that would unequivocally be able to explain the development, presence, or perpetuation of the individual myofascial trigger point or points. Hubbard (1993) found spontaneous needle-EMG activity in the myofascial trigger points, which he explained on the basis of the contraction of the intrafusal muscle fibers. Less convincing were studies that examined local oxygen levels, tissue damage, and sympathetic activity in those muscle believed to harbor a trigger point (Bengtsson et al., 1986, 1989; Bennett, 1989; Boissevian and McCain, 1991; Lund et al., 1986; Simons, 1988; Yunas et al., 1981). The myofascial trigger point (MTrP) is described as a small area (0.5–1 cm) of muscle that is hypersensitive to pressure and is noticeably different from the surrounding region when palpated. In normal muscle, a MTrP cannot be demonstrated and pain cannot be elicited with normal palpatory pressure. Characteristics of a muscle with a MTrP are weakness of contraction but no atrophy. Contraction leads to decreased motility of the corresponding joint (Travell, 1981). This may be of importance for documentation requirements, for instance, in the patient’s medical record, as not only must the incriminated MTrP be adequately identified but also any noticeable functional changes, such as associated loss of joint range of motion or specific muscle weakness, or particular objectively verifiable functional deficits (standing, reading, etc.). The active MTrP has a low threshold for mechanical stimulation. With normal physiological motion, local pain appears, but it is the referred pain in the reference musculature and fascia that is perceived by the patient as symptomatic. The clinically nonsymptomatic latent MTrP refers pain only when a certain amount of palpatory pressure is applied or when an acupuncture needle is inserted into the MTrP (Melzack, 1981). The primary MTrP develops independently and not as the result of trigger point activity elsewhere (Rachlin, 1994). Secondary MTrPs may develop in antagonistic muscles and neighboring protective muscles as the result of stress and muscle spasms (Rachlin, 1994). We have also observed secondary MTrPs to develop in synergistic muscles or muscles of the same functional group, that is, in either the phasic or tonic muscles when one or the other is affected. It is common for patients to experience the pain of a secondary trigger point after a primary trigger point is eliminated (Rachlin, 1994). Satellite MTrPs can develop in the area of the referred pain as the result of persistent resting motor unit activity in the muscle (Travell and Simons, 1983a,b). For instance, a MTrP in the sternal portion of the sternocleidomastoid muscle can cause satellite MTrPs in the sternalis muscle, pectoralis muscle, and the serratus anterior major muscle. Little is known about the etiology of the MTrP, but the list of the many proposed causes includes direct muscle or joint injury, chronic muscle overload, or lengthy periods of hypothermia (Travell, 1981). Latent MTrPs can be activated by small impulses, such as the overstretching of a muscle, a sudden burst of muscle activity associated with overload, or immobilization. The development of MTrPs in the segmental musculature has been known to occur with compression of the nerve root (Travell, 1976). As a rule, they are not reversible, even after successful operative management (e. g., postlaminectomy syndrome) (Travell, 1976). There are reports that joint motion restriction (hypomobility) in the presence of a segmental or somatic dysfunction or inflammatory processes can contribute to the formation of a MTrP in a muscle or exacerbate it (Reynolds, 1981). Fig. 6.4 Examples of trigger points and referred pain in the reference sites (pain reference pattern; after Travell and Simons, 1983a). Referred pain, in the presence of either an active or a latent MTrP, is usually very well-localized by the patient. Using palpation, the painful muscle or part of the muscle (“myotenone”, for definition see below) can be demarcated. The MTrP is described as a “palpable band” (Simons, 1975, 1976b), which should be palpated perpendicular to the direction of the incriminated muscle fibers (similar to the “myotendinoses” also defined below). Palpation often causes a local, painful twitch response in the muscle involved (Simons, 1976a; Travell, 1952). A MTrP may cause pain in one or in several reference sites (Fig. 6.4). The induction of several so-called satellite MTrPs by a primary MTrP is described by Travell (1981). For instance, a MTrP in the sternal region of the sternocleidomastoid muscle can cause satellite MTrPs in the sternalis muscle, pectoralis muscle, and the serratus anterior major muscle. MTrPs can be found in any skeletal muscle; in orthopedic practice, a set of common muscles has been regularly encountered and described (Rachlin, 1994): 1. Posterior neck muscles 2. Sternocleidomastoid muscle and scalene muscles 3. Trapezius muscle (the most commonly encountered MTrP in general) 4. Infraspinatus muscle 5. Supraspinatus muscle 6. Levator scapulae muscle, rhomboid muscles, and thoracic erector spinae muscles 7. Lumbar paraspinal muscles and quadratus lumborum muscle 8. Gluteal muscles (minimus, medius, maximus muscles), tensor fasciae latae, piriformis muscle 9. Rectus femoris muscle and vastus medialis muscle 10. Flexor and extensor muscles of the forearm (“golfer’s elbow” and “tennis elbow” syndromes) 11. Pectoralis minor and major muscles 12. Interossei foot muscles. Other muscles have also been identified as occurring more commonly in the presence of headaches and facial pain. In addition to causing referred pain, the active MTrP can influence autonomic functions in the reference site, including cooling by local vasoconstriction or via increased perspiration and amplified pilomotor activity. Occasionally, hyperalgesic skin regions have been observed (Travell, 1981). In general, and in addition to standard pharmacologic care (e. g., nonsteroidal anti-inflammatories [NSAIDS], analgesics), the activity of the MTrP can be addressed specifically in three ways: (1) introduction of maximal stretch with or without a cooling spray; (2) injection or “dry-needling” of the trigger points; and (3) specific manual medicine techniques applied to treat the MTrP to the incriminated muscle or muscles. The “spray-and-stretch” method is commonly used to treat the MTrP (Travell, 1981). With the help of a cooling spray (e. g., fluoromethane), afferent nociceptive impulses of the skin can be invoked, which in turn appear to reflexly reduce the muscle’s resistance to stretch, thereby facilitating the lengthening of the shortened muscle in the direction of the normal resting length. This “stretch stimulus” appears to be able to reduce the activity of the MTrP, presumably via the reflex pathways within the spinal cord, or possibly in higher central nervous system (CNS) centers. The MTrP activity can be reduced or even extinguished by injection of local anesthetics such as procaine or lido-caine. The muscle that harbors the incriminated MTrP should be carefully stretched passively during the injection. Another form of treatment entirely avoids the need for any injectant by approaching the MTrP simply using an injection needle, a course of treatment known as dry needling.
Referred Pain
Myofascial Pain Syndromes
Myofascial Trigger Points
Palpation of Myofascial Trigger Points
Types of Myofascial Trigger Points
Myofascial Trigger Points and Pain Referral Patterns
Myofascial Trigger Points: Treatment Options
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree